2025-04-17 ゲーテ大学
<関連情報>
- https://aktuelles.uni-frankfurt.de/english/neurodegenerative-disease-als-cellular-repair-system-could-prevent-protein-aggregation/
- https://www.nature.com/articles/s41589-025-01886-4
SUMO-ユビキチンネットワークを介してTDP-43を凝集から保護するPMLへの近接性の誘導 Induced proximity to PML protects TDP-43 from aggregation via SUMO–ubiquitin networks
Kristina Wagner,Jan Keiten-Schmitz,Bikash Adhikari,Upayan Patra,Koraljka Husnjak,François McNicoll,Dorothee Dormann,Michaela Müller-McNicoll,Georg Tascher,Elmar Wolf & Stefan Müller
Nature Chemical Biology Published:17 April 2025
DOI:https://doi.org/10.1038/s41589-025-01886-4
Abstract
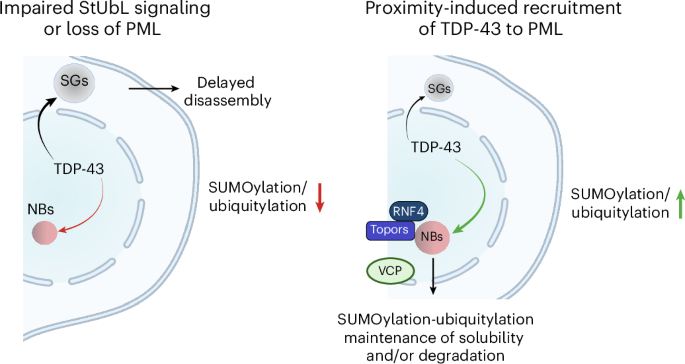
The established role of cytosolic and nuclear inclusions of TDP-43 in the pathogenesis of neurodegenerative disorders has multiplied efforts to understand mechanisms that control TDP-43 aggregation and has spurred searches for approaches limiting this process. Formation and clearance of TDP-43 aggregates are controlled by an intricate interplay of cellular proteostasis systems that involve post-translational modifications and frequently rely on spatial control. We demonstrate that attachment of the ubiquitin-like SUMO2 modifier compartmentalizes TDP-43 in promyelocytic leukemia protein (PML) nuclear bodies and limits the aggregation of TDP-43 in response to proteotoxic stress. Exploiting this pathway through proximity-inducing recruitment of TDP-43 to PML triggers a SUMOylation–ubiquitylation cascade protecting TDP-43 from stress-induced insolubility. The protective function of PML is mediated by ubiquitylation in conjunction with the p97 disaggregase. Altogether, we demonstrate that SUMO–ubiquitin networks protect cells from insoluble TDP-43 inclusions and propose the functionalization of PML as a potential future therapeutic avenue countering aggregation.