2025-05-15 医薬基盤・健康・栄養研究所
<関連情報>
- https://www.nibn.go.jp/pr/press/20250515.html
- https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1574955/full
- https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1574958/full
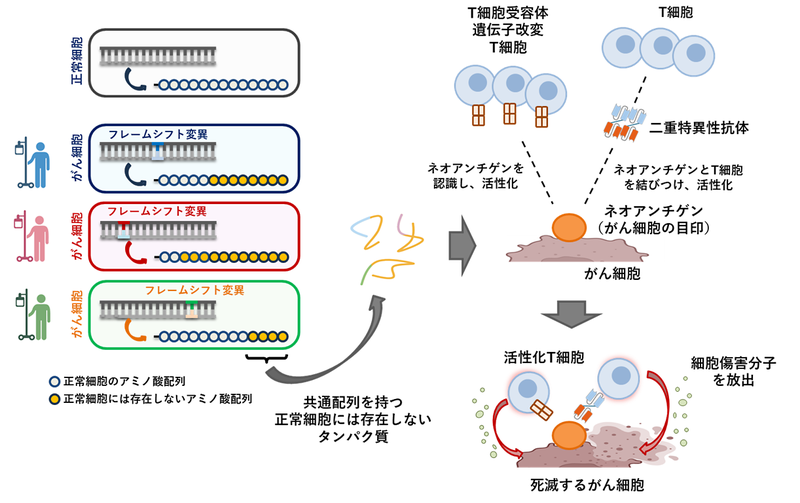
APC遺伝子のフレームシフト変異に由来する共有ネオアンチゲンの同定 Identification of shared neoantigens derived from frameshift mutations in the APC gene
Peng Zhao,Clara Effenberger,Saki Matsumoto,Toshihiro Tanaka,Yusuke Nakamura,Kazuma Kiyotani
Frontiers in Immunology Published:15 May 2025
DOI:https://doi.org/10.3389/fimmu.2025.1574955
Recent advances of cancer immunotherapy have identified neoantigens as essential targets for personalized treatments. However, since neoantigens are generally unique in individual cancers, neoantigen therapies that are more broadly applicable are eagerly awaited. Shared neoantigens, derived from recurrent mutations found across multiple patients, are considered to be a challenging, but promising approach. Here we analyzed shared frameshift neoantigens derived from frameshift indels in TCGA exome sequencing data and identified 760 possible recurrent frameshift mutation clusters (FSCs) that share frameshifted open reding frames and premature stop codons. Among them, we investigated FSCs in the APC gene (APC-F2-1472* and APC-F3-1512*) and identified HLA-A*24:02-restricted frameshift neoantigen peptides that elicited specific CD8+ T cell responses. Subsequently we identified their corresponding T cell receptor (TCR) sequences and generated genetically-engineered T cells expressing these APC frameshift neoantigen-specific TCRs. These engineered T cells specifically recognized target cells presenting these neoantigens and cytotoxic activity against them, supporting the therapeutic potential of targeting APC frameshift neoantigens in cancer immunotherapy. This study provides compelling evidence for the development of neoantigen-based therapies targeting common frameshift peptides, offering a promising approach for more effective, relatively broadly applicable immunotherapeutic strategies that could benefit a subset population of cancer patients.
APCの共有インデルに由来するネオアンチゲンを標的とする二重特異性抗体 Bispecific antibody targeting shared indel-derived neoantigen of APC
Clara Effenberger,Xiaojing Wu,Peng Zhao,Saki Matsumoto,Yusuke Nakamura,Kazuma Kiyotani
Frontiers in Immunology Published:15 May 2025
DOI:https://doi.org/10.3389/fimmu.2025.1574958
T cells play a pivotal role in cancer immunotherapy by recognizing tumor-specific neoantigens presented on HLA molecules, which are specifically expressed on cancer cells. While neoantigens are generally unique to individual cancers, certain neoantigens, known as ‘shared neoantigens’ that are common in a subset of cancer patients, represent promising immunotherapeutic targets. We previously identified an immunogenic shared frameshift neoantigen, 1472SP2, derived from recurrent frameshift indel mutation cluster (APC-F2-1472*) in the APC gene and presented on HLA-A24:02. In this study, we attempted to identify an antibody targeting a complex formed by the APC 1472SP2 neoantigen and HLA-A24. Using the phage display library screening, we isolated single-chain variable fragments (scFvs) that specifically recognize the 1472SP2/HLA-A24 complex. We then designed a bispecific antibody (BsAb) that would connect T cells via an anti-CD3 scFv to the cancer-specific 1472SP2 presented on the HLA-A24 molecule. ELISA analysis revealed that BsAb specifically recognized both 1472SP2-HLA-A24 monomer and CD3 protein. When T cells were co-cultured with antigen-presenting cells expressing HLA-A24:02, IFN-γ release and cytotoxicity were observed only in the presence of 1472SP2-BsAb, indicating that the 1472SP2-BsAb effectively activated T cells to lyse target cells presenting this neoantigen. This approach implies an off-the-shelf, cancer selective approach to target cancers expressing shared neoantigens for patients who are difficult to treat with conventional therapies.