2025-05-12 ユニバーシティ・カレッジ・ロンドン(UCL)
<関連情報>
- https://www.ucl.ac.uk/news/2025/may/scientists-film-heart-forming-3d-earlier-ever
- https://www.embopress.org/doi/full/10.1038/s44318-025-00441-0
哺乳類の胃形成期における細胞移動と心臓運命決定の早期調整 Early coordination of cell migration and cardiac fate determination during mammalian gastrulation
Shayma Abukar, Peter A Embacher, Alessandro Ciccarelli, Sunita Varsani-Brown, Isabel G W North, Jamie A Dean, James Briscoe, and Kenzo Ivanovitch
The EMBO Journal Published:13 May 2025
DOI:https://doi.org/10.1038/s44318-025-00441-0
Abstract
During gastrulation, mesodermal cells derived from distinct regions are destined to acquire specific cardiac fates after undergoing complex migratory movements. Here, we used light-sheet imaging of live mouse embryos between gastrulation and heart tube formation to track mesodermal cells and to reconstruct lineage trees and 3D migration paths for up to five cell divisions. We found independent progenitors emerging at specific times, contributing exclusively to left ventricle/atrioventricular canal (LV/AVC) or atrial myocytes. LV/AVC progenitors differentiated early to form the cardiac crescent, while atrial progenitors later generated the heart tube’s Nr2f2+ inflow tract during morphogenesis. We also identified short-lived multipotent progenitors with broad potential, illustrating early developmental plasticity. Descendants of multipotent progenitors displayed greater dispersion and more diverse migratory trajectories within the anterior mesoderm than the progeny of uni-fated progenitors. Progenitors contributing to extraembryonic mesoderm (ExEm) exhibited the fastest and most dispersed migrations. In contrast, those giving rise to endocardial, LV/AVC, and pericardial cells showed a more gradual divergence, with late-stage behavioural shifts: endocardial cells increased in speed, while pericardial cells slowed down in comparison to LV/AVC cells. Together, these data reveal patterns of individual cell directionality and cardiac fate allocation within the seemingly unorganised migratory pattern of mesoderm cells.
Synopsis
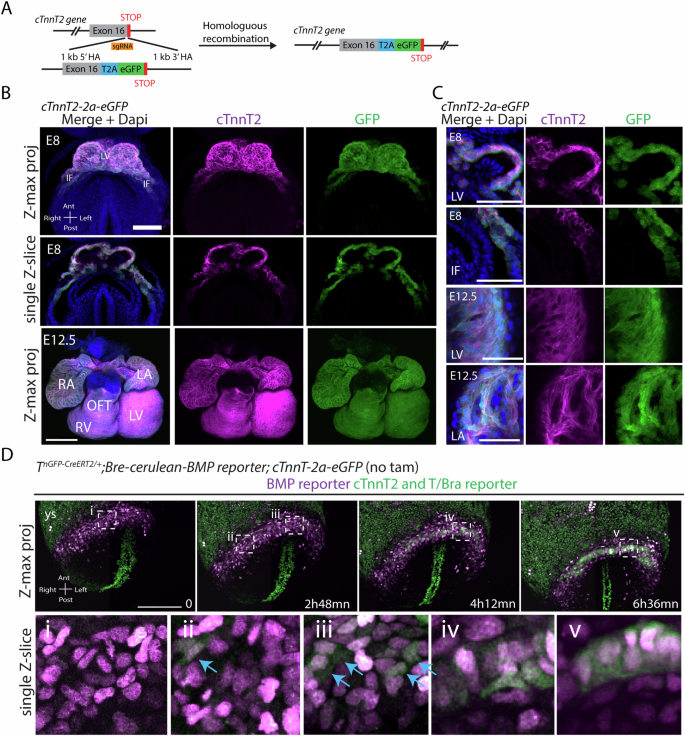
Progenitor cells that will contribute to formation of specific heart tissues are derived from distinct regions of the primitive streak in the developing mouse embryo. This study utilizes light-sheet microscopy in mouse embryos to track individual mesodermal cells destined to the heart for up to 40 h, revealing dynamic behaviours and lineages from gastrulation to heart tube formation.
•The left ventricle progenitors originate from early proximal mesoderm, while atrial progenitors are derived from late proximal mesoderm.
•Left ventricle progenitors differentiate early into myocytes, forming the cardiac crescent, while atrial progenitors differentiate later to establish the heart tube’s Nr2f2+ inflow regions.
•Most early proximal mesodermal cells are uni-fated and contribute predominantly to myocytes.
•Multi-potent progenitors are present and rapidly commit to specific cardiac fates as they migrate toward defined embryonic regions.
•Pairs of sister cells generated by uni-fated progenitors exhibit more coordinated migration paths compared to descendants of multi-potent progenitors.

