2025-05-28 東京科学大学
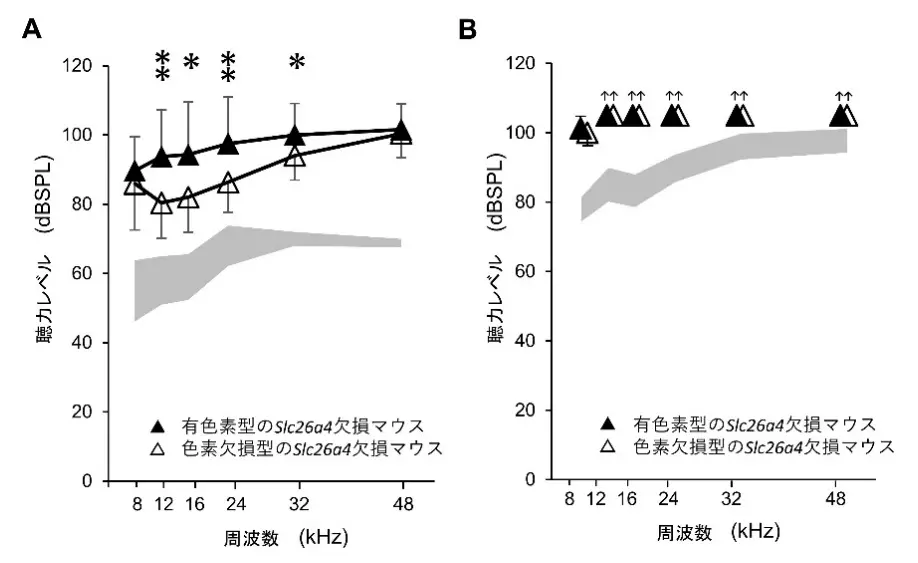
図1. 聴力評価
<関連情報>
- https://www.isct.ac.jp/ja/news/p4aupaqjvt1l#top
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=1611&prevId=&key=922ff140daf70c23f9586a53cf9753c6.pdf
- https://www.sciencedirect.com/science/article/pii/S0969996125001780
SLC26A4欠損マウスにおける難聴に対するメラニンとマクロファージ活性化の影響 Influence of melanin and macrophage activation on hearing loss in SLC26A4 deficient mice
Natsuki Aoki, Ayako Maruyama, Toru Miwa, Natsuko Kurata, Keiji Honda, Yoshiyuki Kawashima, Takeshi Tsutsumi, Taku Ito
Neurobiology of Disease Available online: 17 May 2025
DOI:https://doi.org/10.1016/j.nbd.2025.106962
Highlights
- Albino Slc26a4Δ/Δ mice exhibit less severe hearing loss compared to pigmented Slc26a4Δ/Δ mice.
- Macrophage morphology, including size and structure, differs significantly in the stria vascularis of pigmented mice.
- Melanin production may exacerbate hearing loss, potentially through complex interactions with oxidative stress and immune responses.
- Microarray analysis reveals altered gene expression patterns associated with melanin synthesis and cellular responses.
- Genetic background and pigmentation are critical factors influencing both disease progression and potential treatment strategies.
Abstract
Hearing loss associated with SLC26A4 mutations exhibits diverse phenotypes, including congenital, acquired, progressive, and fluctuating impairments. This study investigates how pigmentation influences auditory dysfunction and immune responses in the stria vascularis using albino and pigmented Slc26a4 knockout (Slc26a4Δ/Δ) mice. We found that albino Slc26a4Δ/Δ mice exhibited significantly less severe hearing loss along with reduced macrophage activation than their pigmented counterpart did. Three-dimensional morphometric analyses revealed that macrophages in pigmented Slc26a4Δ/Δ mice were significantly larger, more rounded, and structurally distinct from those in albino mice, suggesting divergent activation states. PHATE-based trajectory analysis further confirmed that these macrophages follow separate activation pathways influenced by pigmentation. Transcriptomic profiling showed no upregulation of melanogenesis-related genes, implying that melanin accumulation arises from impaired degradation rather than increased synthesis. Functional enrichment analysis highlighted dysregulation in proteolysis and phosphate metabolism, implicating metabolic stress and chronic inflammation in disease progression. Based on these findings, we propose a multi-step pathophysiological cascade in which SLC26A4 deficiency leads to metabolic imbalance, pathological melanin accumulation, macrophage activation, and ultimately, progressive hearing loss. This work reveals a context-dependent dual role of melanin and demonstrates how genetic background and pigmentation influence immune responses and cochlear pathology. These insights may inform future strategies for individualized approaches to diagnosis and therapy in SLC26A4-related hearing loss.