2025-06-01 北京大学(PKU)
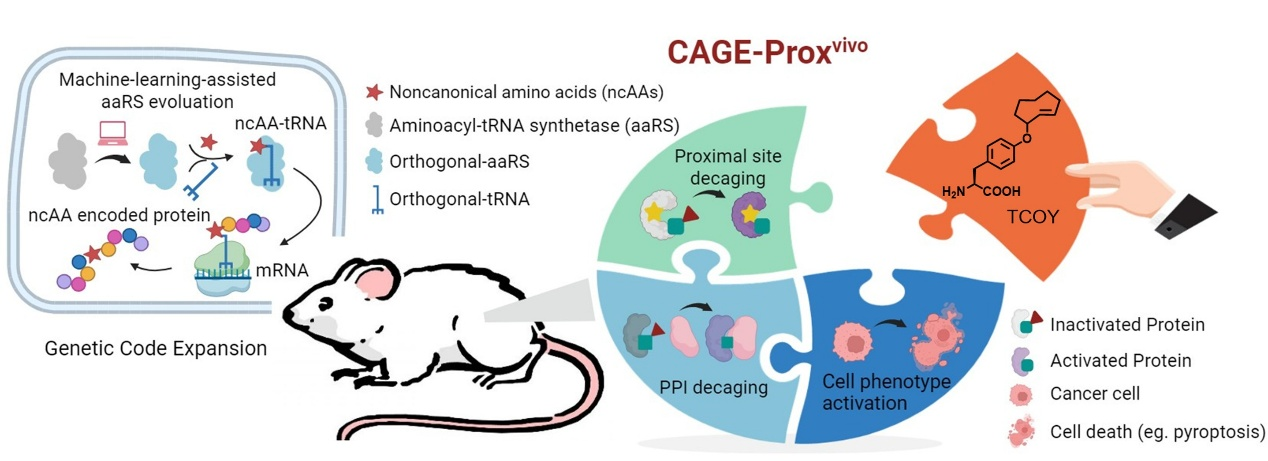 CAGE-Proxvivo technology: Trans-cyclooctene-caged tyrosine (TCOY) is site-specifically incorporated into a protein via machine-learning-assisted genetic code expansion, temporarily masking protein activity. The protein is then reactivated through IEDDA reaction-mediated bioorthogonal decaging within its catalytic pocket in vivo in mice.
CAGE-Proxvivo technology: Trans-cyclooctene-caged tyrosine (TCOY) is site-specifically incorporated into a protein via machine-learning-assisted genetic code expansion, temporarily masking protein activity. The protein is then reactivated through IEDDA reaction-mediated bioorthogonal decaging within its catalytic pocket in vivo in mice.
<関連情報>
- https://newsen.pku.edu.cn/news_events/news/research/14964.html
- https://www.cell.com/cell/abstract/S0092-8674(25)00517-3
生きたマウスにおける機械学習支援による万能タンパク質の活性化 Machine-learning-assisted universal protein activation in living mice
Xin Wang ∙ Yuan Liu ∙ Zhenchao Wang ∙ … ∙ Xinyuan Fan ∙ Chu Wang ∙ Peng R. Chen
Cell Published:May 27, 2025
DOI:https://doi.org/10.1016/j.cell.2025.05.006
Highlights
•A machine-learning-assisted aaRS evolution for unnatural amino acid incorporation
•A universal platform for modulating protein activity and interactions in living animals
•Tumor-cell-specific activation of pyroptosis for stimulating antitumor immunity
•Bioorthogonally gated anti-CD3 antibody for chemically controlled T cell engagement
Summary
A universal strategy to precisely control protein activation in living animals is crucial for gain-of-function study of proteins under in vivo settings. We herein report CAGE-Proxvivo, a computer-aided proximal decaging strategy for on-demand protein activation as well as protein-protein interaction modulations in living mice. Through machine-learning-assisted evolution of desired aminoacyl-tRNA synthetases (aaRSs), we successfully incorporated chemically caged amino acids into rationally designed “decaging sites” to transiently block target proteins’ function, which can be restored in situ via a small-molecule-triggered bioorthogonal cleavage reaction. This method demonstrates broad applicability ranging from activating proteins of interest to cell-type-specific modulation of distinct phenotypes in living systems. Beyond the active-pocket decaging, CAGE-Proxvivo also enables precise control of protein-protein interactions, as exemplified by a “gated” anti-CD3 antibody that permits chemically regulated T cell recruitment and activation at tumor sites. Overall, CAGE-Proxvivo offers a universal platform for time-resolved biological studies and on-demand therapeutic interventions under living conditions.