2025-06-06 スイス連邦工科大学ローザンヌ校(EPFL)
Illustration of the Multivalent Evolved DNA-based SUpramolecular Assemblies (MEDUSA), shown in white, interacting with a target protein (pink). 2025 PBL EPFL CC BY SA 4.0
<関連情報>
- https://actu.epfl.ch/news/ultra-selective-aptamers-give-viruses-a-taste-of-t/
- https://www.nature.com/articles/s41565-025-01939-8
標的特異的空間構造を有するアプタマーの多価超分子集合体の進化 Evolution of multivalent supramolecular assemblies of aptamers with target-defined spatial organization
Artem Kononenko,Vincenzo Caroprese,Yoan Duhoo,Cem Tekin & Maartje M. C. Bastings
Nature Nanotechnology Published:06 June 2025
DOI:https://doi.org/10.1038/s41565-025-01939-8
Abstract
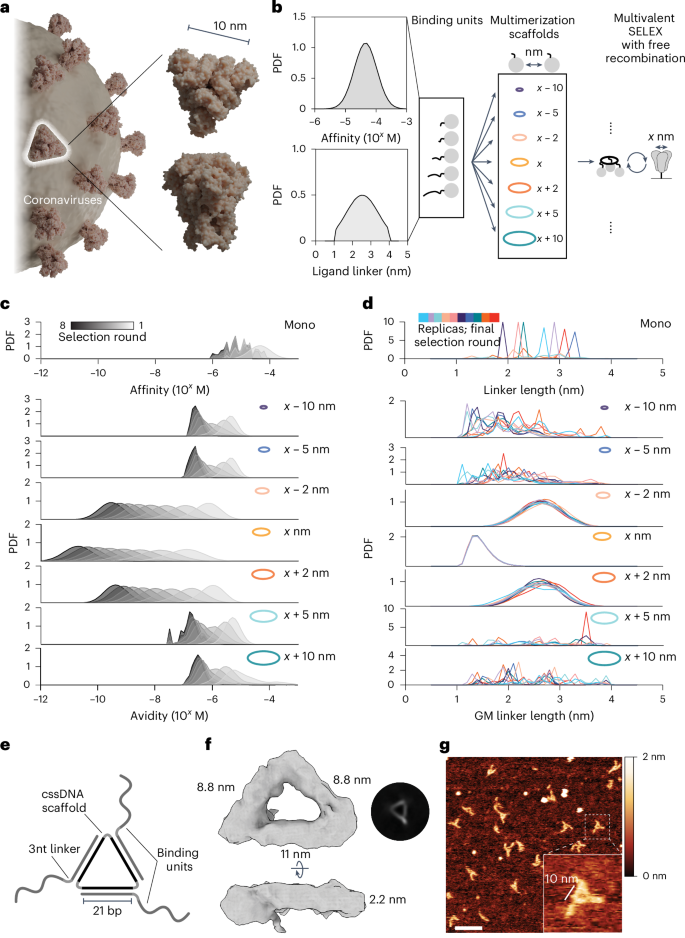
Rapid identification of neutralizing molecules against new and mutating viruses is key to efficiently combating biorisk. Current binder identification techniques use a monovalent library of potential binders. Interestingly, proteins on pathogens are often homo-oligomeric—for example, the SARS-CoV-2 spike protein is a homotrimer. Here we describe a simple strategy, MEDUSA (multivalent evolved DNA-based supramolecular assembly), to evolve multivalent assemblies of aptamers with precise interligand spacing and three-fold symmetry, mirroring the geometric structure of many viral capsid proteins. MEDUSA allowed the selection of potent SARS-CoV-2 spike binders structurally distinct from any known aptamers. Decoupling the geometric and structural rigidity contributions toward selectivity made it possible to connect form to function, as demonstrated by the design of tunable fluorescent sensors. This approach offers a blueprint for targeting geometrically defined pathogen structures and developing rapid-response tools for emerging pathogens.


