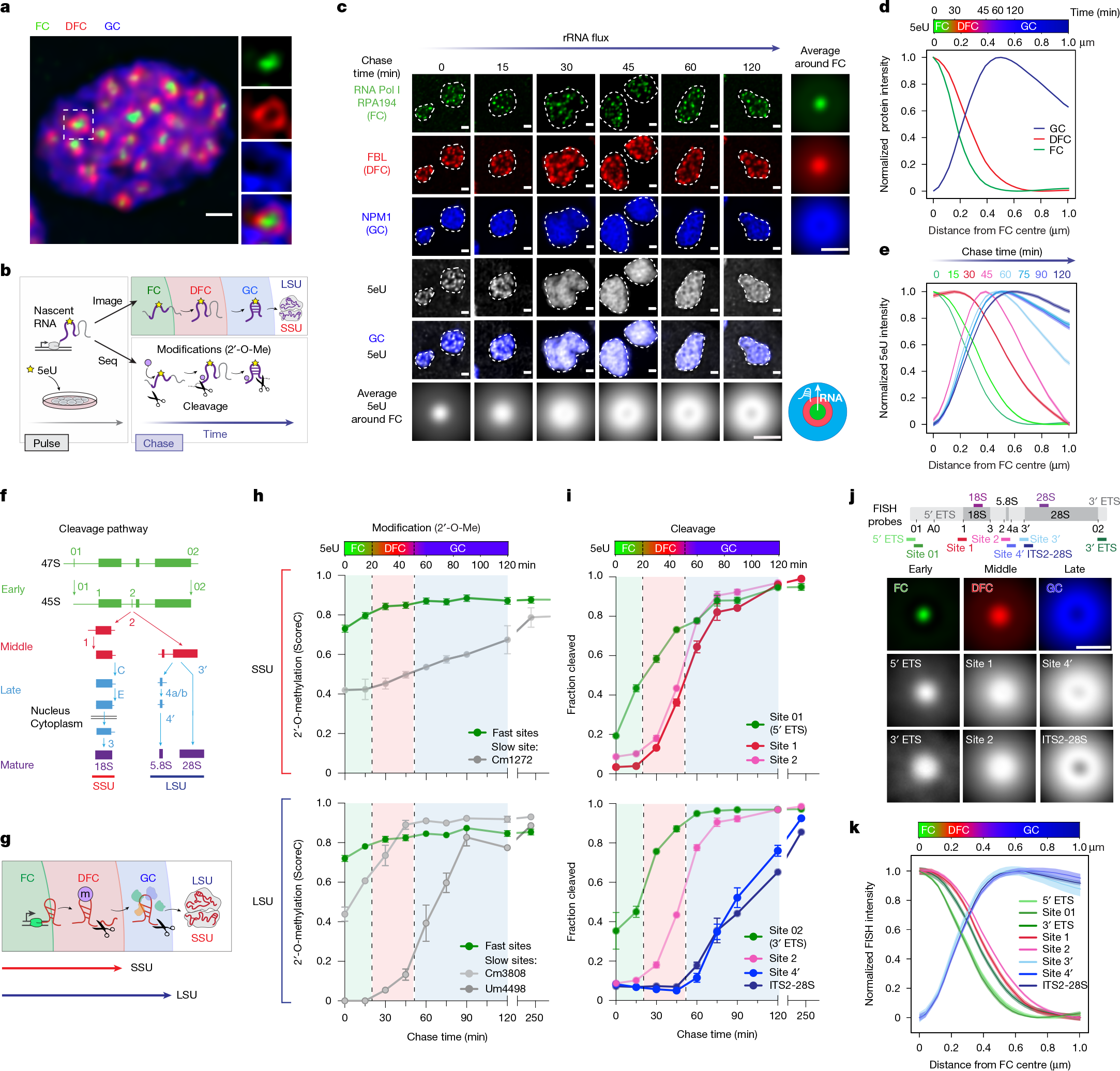
2025-07-18 九州大学
<関連情報>
- https://www.kyushu-u.ac.jp/ja/researches/view/1296
- https://www.kyushu-u.ac.jp/f/62513/25_0718_01.pdf
- https://www.nature.com/articles/s41467-025-61662-9
脱アミノ官能基化のための立体障害アミンからの触媒的ジアゼン合成 Catalytic diazene synthesis from sterically hindered amines for deaminative functionalization
Taro Tsuji,Isora Fukumoto,Takara Hario,Mikihiro Hayashi,Ayumi Osawa,Takashi Ohshima & Ryo Yazaki
Nature Communications Published:07 July 2025
DOI:https://doi.org/10.1038/s41467-025-61662-9
Abstract
Primary amines are highly ubiquitous functional groups found in diverse natural products and building blocks. Despite their widespread application as nucleophiles, the potential for facile deaminative functionalization utilizing primary amines, particularly sterically hindered α-tertiary amines, has remained less explored. Herein, we report catalytic direct synthesis of aliphatic diazenes from sterically hindered α-tertiary amines. The catalytic diazene synthetic method exhibits wide functional group tolerance, allowing for expeditious access to a wide array of hitherto-inaccessible, highly congested diazenes in a short time. Noteworthy is that the present catalytic method enables the synthesis of various hetero-diazenes using distinct α-tertiary amines in just one step for the first time. The catalytic process could also be readily incorporated into Fmoc solid-phase peptide synthesis, enabling the synthesis of elastin-derived diazene, which contains 12 amino acid residues. The catalytic diazene synthetic method enables efficient deaminative transformation of C–N bonds into C–halogen, C–H, C–O, C–S, C–Se, and C–C bonds through carbon-centered radical formation.