2025-07-25 愛媛大学
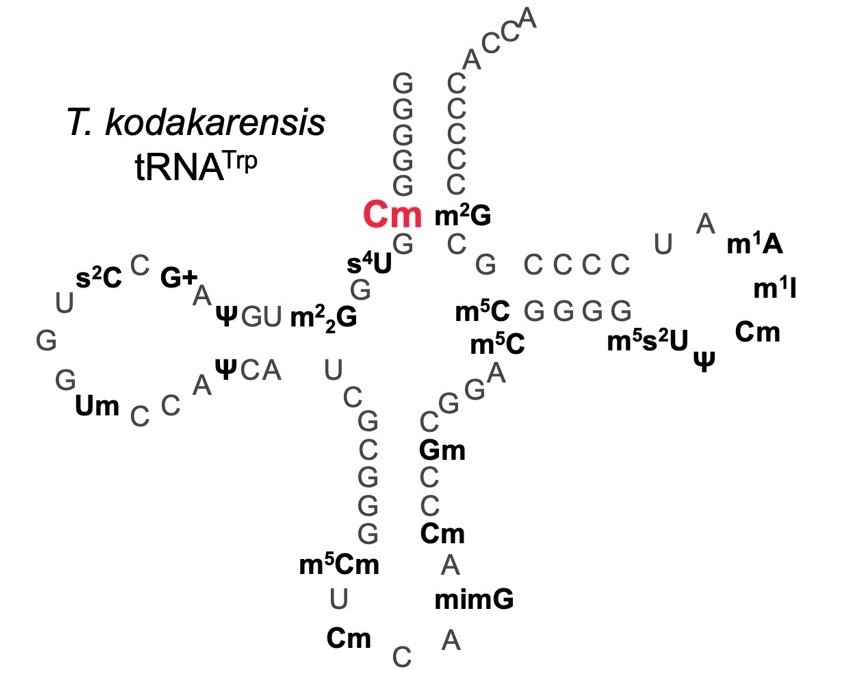 Thermococcus kodakarensis tRNATrpの二次構造
Thermococcus kodakarensis tRNATrpの二次構造
<関連情報>
- https://www.ehime-u.ac.jp/data_relese/pr_20250725_eng/
- https://www.ehime-u.ac.jp/wp-content/uploads/2025/07/pr_20250725_eng.pdf
- https://academic.oup.com/nar/article/53/13/gkaf579/8198049
A transfer RNA methyltransferase with an unusual domain composition catalyzes 2′-O-methylation at position 6 in tRNA 構造も機能も型破り!これまでにないドメイン構成を持つ tRNA メチル化酵素を発見
Teppei Matsuda , Ryota Yamagami , Aoi Ihara , Takeo Suzuki , Akira Hirata , Hiroyuki Hori
Nucleic Acids Research Published:12 July 2025
DOI:https://doi.org/10.1093/nar/gkaf579
Abstract
Thermococcus kodakarensis tRNATrp contains 2′-O-methylcytidine at position 6 (Cm6). However, the tRNA methyltransferase responsible for the modification has not been identified. Using comparative genomics, we predicted TK1257 as a candidate gene for the modification enzyme. Biochemical and mass spectrometry studies of purified recombinant TK1257 gene product demonstrated that it possesses a tRNA methyltransferase activity for Cm6 formation. This protein has a highly unusual composition of domains, containing N-terminal ferredoxin-like, SPOUT catalytic, and THUMP domains. Previous to this study, all known THUMP-related tRNA methyltransferases were shown to contain a Rossmann fold catalytic domain and the nucleosides they produced were N2-methylguanosine and/or N2, N2-dimethylguanosine. Therefore, our findings extend the knowledge of architecture of tRNA methyltransferases. We named the TK1257 gene product TrmTS and showed that it can synthesize Am6 and Um6 as well as Cm6. A trmTS gene deletion strain showed slight growth retardation at high temperatures. Site-directed mutagenesis studies revealed catalytically and structurally important amino acid residues in TrmTS and identified a TrmTS-specific linker that is structurally essential. We revealed that TrmTS recognizes the 3′-CCA terminus of tRNA but does not require the three-dimensional structure of tRNA for its activity. Finally, we constructed a model of the binding between TrmTS and tRNA.