2025-07-25 東京科学大学
<関連情報>
- https://www.isct.ac.jp/ja/news/8ulztxqcij84
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=2003&prevId=&key=6ef6572e111e8a1815f9e9cdb72db6ce.pdf
- https://ard.eular.org/article/S0003-4967(25)01590-0/abstract
POS0169 全身性エリテマトーデス患者における疾患活動状態の予測に手首装着型ウェアラブルデバイスを活用する POS0169 PREDICTION OF DISEASE ACTIVITY STATE IN PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS BY UTILIZING WRIST-WORN WEARABLE DEVICES
T. Niwano ∙ T. Yamaguchi ∙ D. Kawata ∙ … ∙ R. Koike ∙ S. Yasuda ∙ T. Hosoya
Annals of the Rheumatic Diseases Published:2025
DOI:https://doi.org/10.1016/j.ard.2025.05.557
Abstract
Background:
Recent drug development and sophisticated treatment regimens have achieved good efficacy and tolerability in the acute phase of patients with systemic lupus erythematosus (SLE), whereas the maintenance treatment regimens are not yet standardized [1]. Based on the principles of treat-to-target, physicians try to assess disease activity at each clinic visit and adapt the pharmacological interventions according to the patient’s backgrounds. However, safe drug tapering/withdrawal and early recognition of relapse signs remain challenging clinical issues. Established clinical indices do not always provide a complete picture of the disease activity. Recent studies have demonstrated that Patient-Reported Outcomes (PROs) may reflect subjective symptoms that cannot be detected by conventional examinations. Recently, wearable devices, such as wrist-worn devices, have become capable of sensing health-related data, such as physical activity, heart rate, and sleep status, non-invasively and continuously. Several studies have already demonstrated the usefulness of these data for management in cardiology and mental health disorders [2, 3]. Additionally, the disease activity in rheumatoid arthritis was associated with sleep quality sensed by wrist-worn devices [4]. Since the nature of SLE is autoimmunity and chronic inflammation, the disease activity must spontaneously and continuously fluctuate, similar to other diseases. We thus considered the possibility of continuously evaluating SLE activity by combining health-related data to PROs.
Objectives:
To develop a predictive model for detecting the achievement of Lupus Low Disease Activity State (LLDAS), one of the potential therapeutic targets.
Methods:
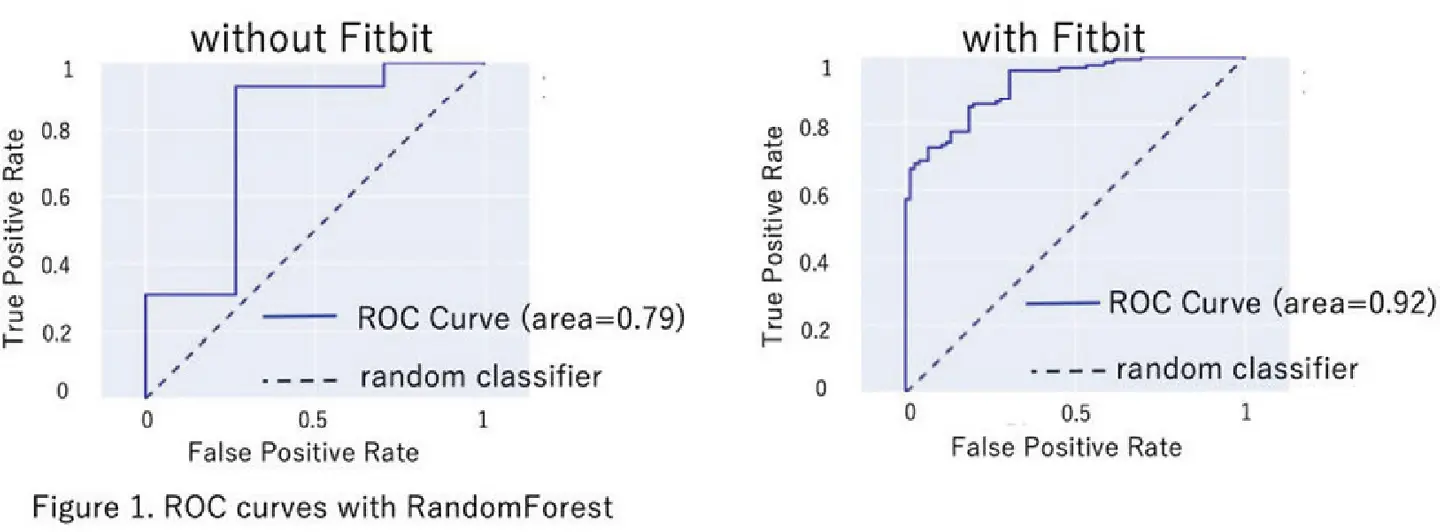
We conducted a cohort study at Tokyo Medical and Dental University Hospital (currently Institute of Science Tokyo Hospital) from July 2023 to September 2024. Patients aged 15 years and older were enrolled with a diagnosis of SLE based on the 1997 ACR revised criteria or the 2019 EULAR/ACR Classification Criteria. We collected treatment histories and laboratory data from medical records. Subjective symptoms were obtained via a visual analog scale and the Lupus PRO questionnaire. The achievement of LLDAS was defined by the physician at the clinical visit. For some patients, a wrist-worn wearable device (Fitbit Inspire 3), was provided according to their preferences. The health-related data, including resting heart rate, sleep status, and step counts, were collected from the SelfBase platform (Tech Doctor, Inc.). Periods with zero step counts were regarded as resting. These data were averaged daily for 7 days before and after the clinical visit. The health-related data were blinded to the physicians during the study period. For machine learning, we used four well-established machine learning Methods: Random Forest, CatBoost, XGBoost, and LGBM.
Results:
Among 274 SLE patients, 112 agreed to wear the Fitbit. While patients’ personal interests in the wearable device might differ based on their background, no difference was found in gender, age, or the achievement of LLDAS between the Fitbit-wearing group and the non-Fitbit-wearing group, indicating that Fitbit wearers in this cohort did not represent a specific subset of population. Then, we investigated whether a prediction model could identify LLDAS achievement. We evaluated two prediction models using machine learning methods. The first model was generated using the combination of “clinical indices + treatment details + PRO,” and the second one incorporated these three variables plus Fitbit features (Table 1 and Figure 1). In all four machine learning methods, the ROC-AUC values were higher when Fitbit features were added, indicating that health-related information increased the prediction accuracy. Notably, SHAP values, which explain how each feature influences the model’s predictions, highlighted the following key features (in order of importance): glucocorticoid dosage on the previous visit, warfarin usage, anti-dsDNA antibody titers, pain domain score from LupusPRO, and resting heart rate value from Fitbit.