2025-09-03 ハーバード大学
<関連情報>
- https://seas.harvard.edu/news/2025/09/upcycling-proteins-just-got-easier
- https://www.nature.com/articles/s41467-025-61959-9#Ack1
エントロピー駆動型変性による迅速なゲル-固体転移を介した持続可能なタンパク質再生 Entropy-driven denaturation enables sustainable protein regeneration through rapid gel-solid transition
Yichong Wang,Junlang Liu,Michael M. Peters,Ryoma Ishii,Dianzhuo Wang,Sourav Chowdhury,Kevin Kit Parker & Eugene I. Shakhnovich
Nature Communications Published:26 July 2025
DOI:https://doi.org/10.1038/s41467-025-61959-9
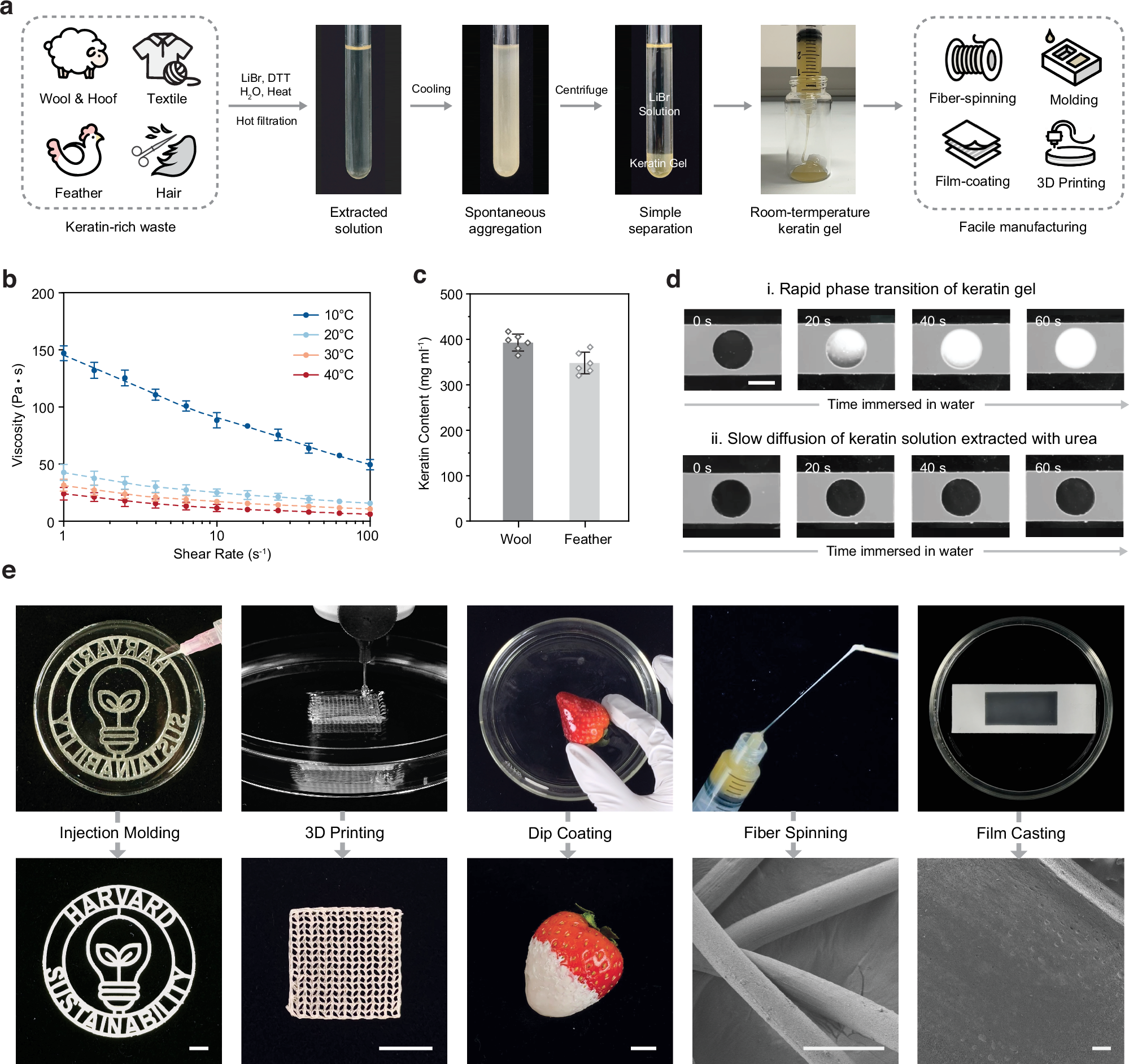
Abstract
The upcycling of protein materials has long been hindered by the difficulty in restructuring them to usable forms. In contrast to proteins extracted using conventional organic denaturants, keratin treated with concentrated inorganic lithium bromide (LiBr) solution undergoes spontaneous aggregation into a stable gel with rapid phase-transition capability. We hypothesize that this distinct behaviour arises from an alternative denaturation mechanism that does not rely on direct interactions between proteins and concentrated ions. To investigate this, we study the denaturation effects of concentrated inorganic ion pairs using thermodynamic and spectroscopic analyses combined with atomistic molecular simulations. Through the isolation of indirect solute effects, our findings suggest a universal mechanism of salt-induced denaturation driven by entropy instead of enthalpy. We find that concentrated ion pairs like LiBr disrupt the water network structure rather than directly interacting with proteins. The mechanistic insight enables us to refine our previous extraction process of keratin materials, allowing for the spontaneous separation of denatured keratin into a condensed gel phase without additional chemicals and achieve closed-loop recycling of the LiBr denaturant. This simple, effective strategy can repurpose protein resources into versatile biomaterials in a simple, effective way without the need to separate organic denaturants from bulk proteins.

