2025-09-10 マサチューセッツ工科大学(MIT)
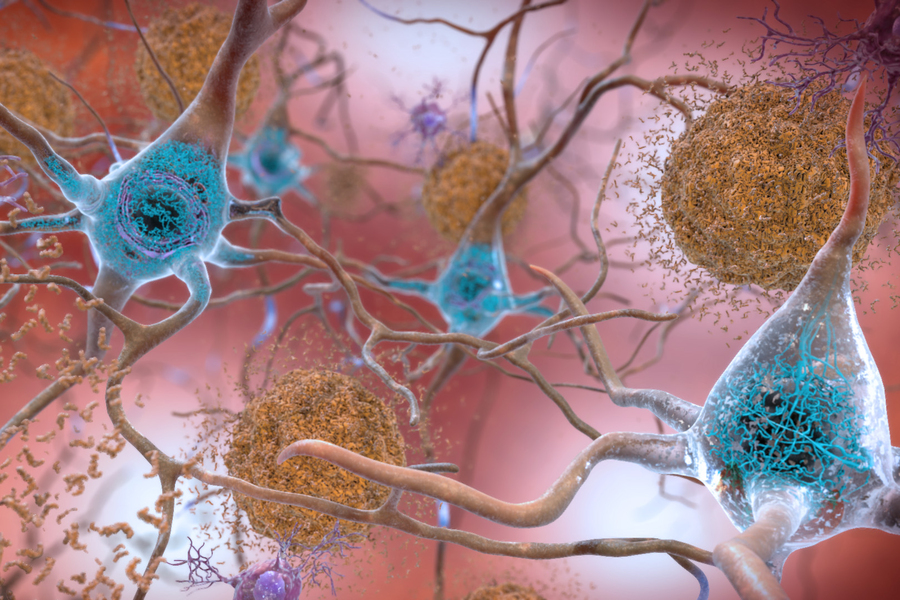
In the Alzheimer’s affected brain, abnormal levels of the beta-amyloid protein clump together to form plaques (seen in brown) that collect between neurons and disrupt cell function. Abnormal collections of the tau protein accumulate and form tangles (seen in blue) within neurons, harming synaptic communication between nerve cells.
Credit: National Institute on Aging, NIH
<関連情報>
- https://news.mit.edu/2025/study-explains-how-rare-gene-variant-contributes-alzheimers-disease-0910
- https://www.nature.com/articles/s41586-025-09520-y
ABCA7変異体は神経細胞内のホスファチジルコリンとミトコンドリアに影響を与える ABCA7 variants impact phosphatidylcholine and mitochondria in neurons
Djuna von Maydell,Shannon E. Wright,Ping-Chieh Pao,Colin Staab,Oisín King,Andrea Spitaleri,Julia Maeve Bonner,Liwang Liu,Chung Jong Yu,Ching-Chi Chiu,Daniel Leible,Aine Ni Scannail,Mingpei Li,Carles A. Boix,Hansruedi Mathys,Guillaume Leclerc,Gloria Suella Menchaca,Gwyneth Welch,Agnese Graziosi,Noelle Leary,George Samaan,Manolis Kellis & Li-Huei Tsai
Nature Published:10 September 2025
DOI:https://doi.org/10.1038/s41586-025-09520-y
Abstract
Loss-of-function variants in the lipid transporter ABCA7 substantially increase the risk of Alzheimer’s disease1,2, yet how they impact cellular states to drive disease remains unclear. Here, using single-nucleus RNA-sequencing analysis of human brain samples, we identified widespread gene expression changes across multiple neural cell types associated with rare ABCA7 loss-of-function variants. Excitatory neurons, which expressed the highest levels of ABCA7, showed disrupted lipid metabolism, mitochondrial function, DNA repair and synaptic signalling pathways. Similar transcriptional disruptions occurred in neurons carrying the common Alzheimer’s-associated variant ABCA7 p.Ala1527Gly3, predicted by molecular dynamics simulations to alter the ABCA7 structure. Induced pluripotent stem (iPS)-cell-derived neurons with ABCA7 loss-of-function variants recapitulated these transcriptional changes, displaying impaired mitochondrial function, increased oxidative stress and disrupted phosphatidylcholine metabolism. Supplementation with CDP-choline increased phosphatidylcholine synthesis, reversed these abnormalities and normalized amyloid-β secretion and neuronal hyperexcitability—key Alzheimer’s features that are exacerbated by ABCA7 dysfunction. Our results implicate disrupted phosphatidylcholine metabolism in ABCA7-related Alzheimer’s risk and highlight a possible therapeutic approach.

