2025-09-30 中山大学(SYSU)
<関連情報>
- https://www.sysu.edu.cn/sysuen/info/1012/57481.htm
- https://www.nature.com/articles/s41586-025-09574-y
SPP1は膵臓癌における間葉系細胞の運命維持に必要である SPP1 is required for maintaining mesenchymal cell fate in pancreatic cancer
Huafu Li,Linxiang Lan,Hengxing Chen,May Zaw Thin,Hari Ps,Jessica K. Nelson,Ian M. Evans,E. Josue Ruiz,Rongjie Cheng,Li Tran,Mark Allen,Jian Ma,Tingzhuang Yi,Chunming Wang,Yulong He,Naomi Guppy,Anguraj Sadanandam,Shao-Zhen Lin,Changhua Zhang & Axel Behrens
Nature Published:24 September 2025
DOI:https://doi.org/10.1038/s41586-025-09574-y
Abstract
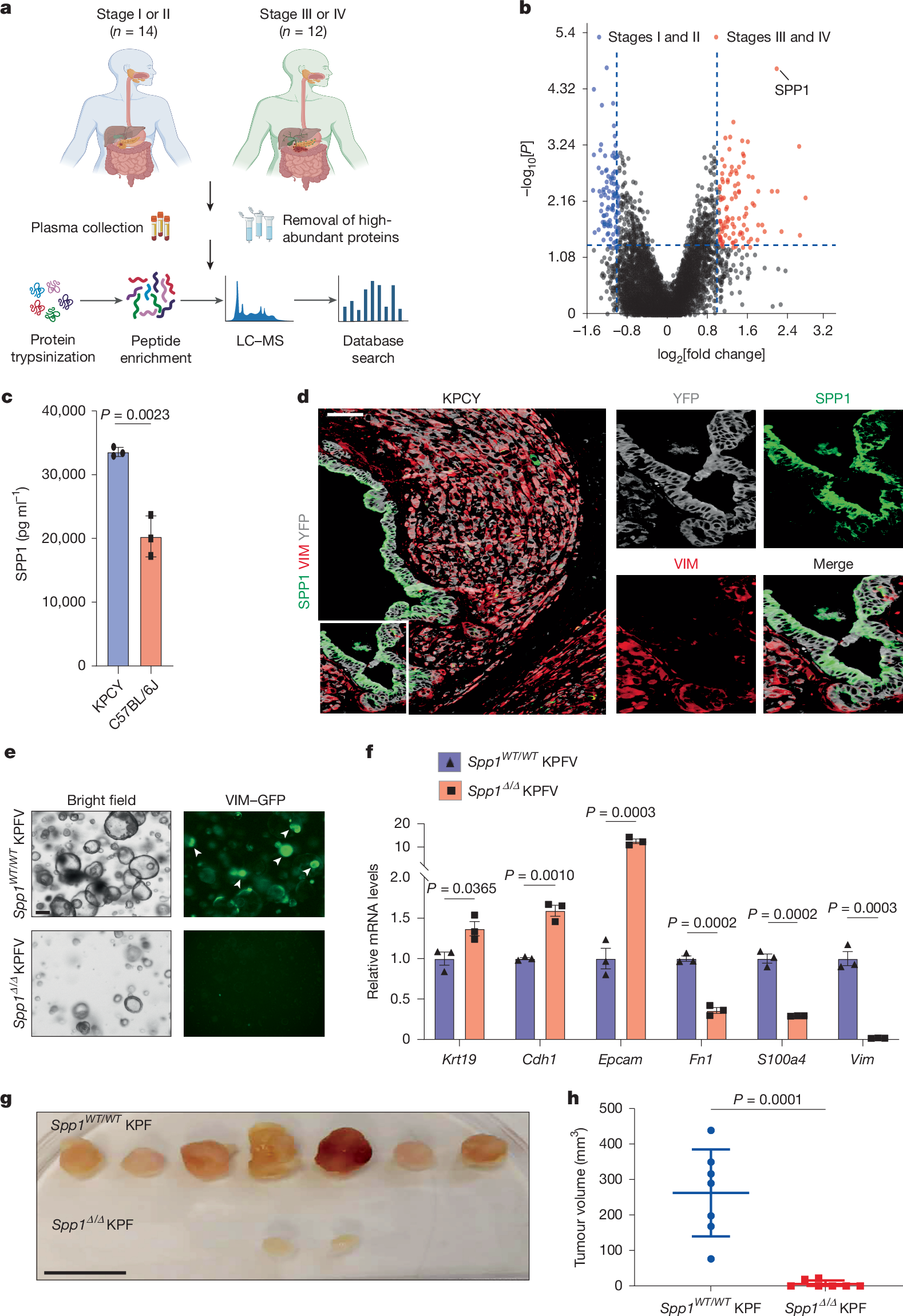
Elucidating the complex network of communication between tumour cells is central to understanding cell fate decisions and progression of pancreatic ductal adenocarcinoma (PDAC)1,2. We previously showed that constant suppression of BMP activity by the BMP antagonist GREM1 secreted by mesenchymal PDAC cells is essential for maintaining the fate of epithelial PDAC cells3. Here we identify SPP1 (also known as osteopontin)4 as a key regulator of mesenchymal cell fate in pancreatic cancer. Proteomic analysis of plasma from patients with PDAC showed that SPP1 is substantially upregulated in late-stage disease. Inactivation of Spp1 led to a delay in tumorigenesis in mouse PDAC models and abolished metastasis formation. Spp1 was expressed in epithelial PDAC cells, and Spp1 inactivation resulted in a conversion of mesenchymal to epithelial PDAC cells. Mechanistically, SPP1 bound the CD61 receptor on mesenchymal PDAC cells to induce Bmp2 and Grem1 expression, and GREM1 inhibition of BMP signalling was required for Spp1 expression in epithelial cells, thereby forming an intercellular regulatory loop. Concomitant inactivation of Grem1 reverted the epithelial phenotype of Spp1 knockout to fully mesenchymal PDAC. Conversely, Grem1 heterozygosity combined with Spp1 knockout resulted in wild-type PDAC histology, a result that confirmed the direct antagonistic functions of these factors. Hence, mesenchymal and epithelial PDAC cell fates are determined by the reciprocal paracrine regulation of the soluble factors GREM1 and SPP1.

