2025-10-17 東京大学,星薬科大学,理化学研究所,JCRファーマ株式会社
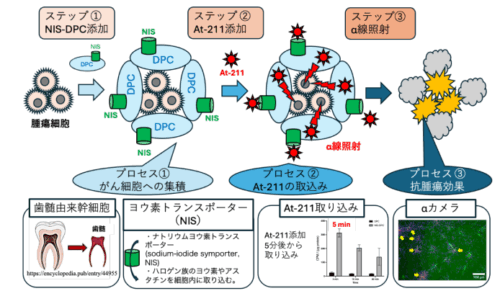
図 1:改良型歯髄由来幹細胞のがん細胞への集積とアスタチン-211 の迅速な取り込みを利用した新規治療法
添加した NIS-DPC 細胞(ステップ①)は、がん細胞へ集積します(プロセス①)。次にアスタチン-211 を添加すると(ステップ②)、がん細胞付近の NIS-DPC 細胞がアスタチン-211 を取り込みます(プロセス②)。取り込まれたアスタチン-211 は α 線を照射し(ステップ③)、周囲に存在する腫瘍細胞を殺傷します(プロセス③)。
<関連情報>
- https://www.u-tokyo.ac.jp/content/400272699.pdf
- https://www.biorxiv.org/content/10.1101/2025.10.15.682497v1
遺伝子組み換え歯髄細胞とアスタチン-211を用いた胃癌腹膜播種に対する新規治療法 Novel therapy for gastric cancer peritoneal dissemination using genetically modified dental pulp cells and astatine-211
Koumei Kuge, Wan-Ying Du, Hiroki Masuda, Tomohiko Yasuda, Toshifumi Tatsumi, Xiaojie Yin, Masao Yoshino, Akira Sugiyama, Ryohei Numata, Wataru Yokoyama, Hidenori Shibahara, Tomoko Tanaka, Mika Kobayashi, Akashi Taguchi, Takahide Kohro, Naoko Takubo, Masahiro Nakamura, Jaewoong Jang, Yoshitaka Kumakura, Hiroshi Yoshida, Akira Yoshikawa, Hiromitsu Haba, Youichiro Wada, Kiwamu Imagawa, Sachiyo Nomura
bioRxiv Posted: October 15, 2025
DOI:https://doi.org/10.1101/2025.10.15.682497
SUMMARY
Peritoneal dissemination of gastric cancer represents a terminal stage with limited therapeutic options and a five-year survival rate below 10%. To develop a more effective treatment, we established a novel α-particle–based approach using genetically engineered human dental pulp stem cells (DPCs) expressing the sodium/iodide symporter (NIS) to deliver astatine-211 (At-211) directly to tumor sites. DPCs, isolated and expanded under differentiation-suppressive conditions, showed strong tumor-homing ability when injected intraperitoneally into a mouse model of gastric cancer dissemination. Fluorescent imaging and histological analysis confirmed selective accumulation of DPCs within tumor lesions through CXCR4/SDF-1–mediated chemotaxis. Introduction of the NIS gene markedly increased SLC5A5 expression and enabled efficient uptake of At-211. High-resolution α-particle imaging visualized α-ray emission specifically from NIS-DPCs, confirming intracellular retention of the radionuclide. In vivo, sequential administration of NIS-DPCs followed by Na[At-211] led to pronounced regression of peritoneal tumors and significant survival extension compared with controls. The therapeutic mechanism involves three coordinated steps: tumor-directed migration of NIS-DPCs, At-211 uptake via NIS transporters, and localized α-particle–mediated cytotoxicity. This study introduces a novel concept of cell-based α-radiotherapy integrating regenerative and nuclear medicine. Given the established clinical safety of DPCs in humans (J-REPAIR, jRCT1080224505), this NIS-DPC platform offers a promising strategy for precise, short-range irradiation of disseminated gastric cancer and potentially other intractable malignancies.

