2025-10-20 東京科学大学
Web要約 の発言:
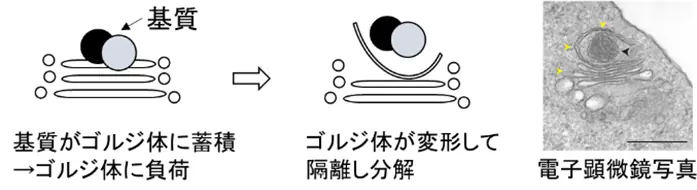
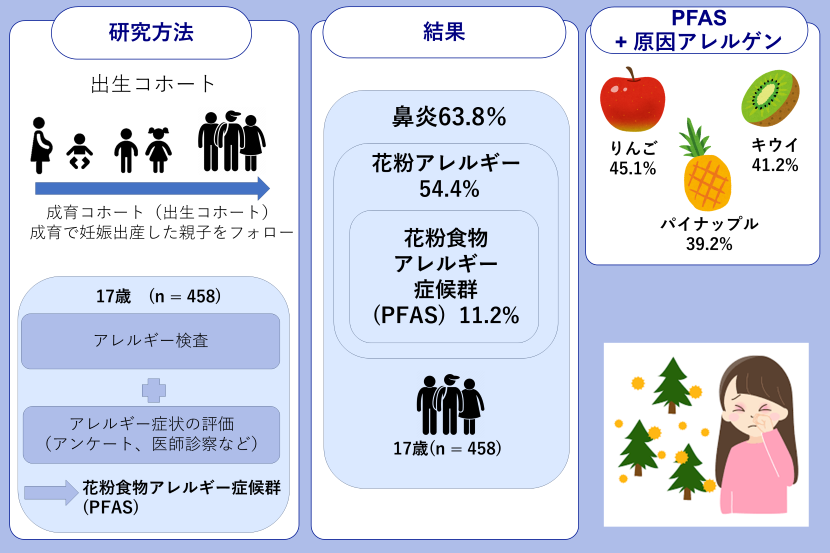
図1. GOMEDの形態学的特徴
<関連情報>
オプチニューリンはゴルジ膜関連分解におけるユビキチン化基質のアダプタータンパク質であるOptineurin is an adaptor protein for ubiquitinated substrates in Golgi membrane-associated degradation
Yoichi Nibe-Shirakihara,Shinya Honda,Satoko Arakawa,Satoru Torii,Hajime Tajima Sakurai,Hirofumi Yamaguchi,Shigeru Oshima,Ryuichi Okamoto,Michael Lazarou,Hideshi Kawakami & Shigeomi Shimizu
Nature Communications Published:20 October 2025
DOI:https://doi.org/10.1038/s41467-025-64400-3
Abstract
Golgi membrane-associated degradation (GOMED) is a process that leading to the degradation of proteins that have passed through the trans-Golgi membranes upon Golgi stress. GOMED is morphologically similar to autophagy, but the substrates degraded are different, and they thus have different biological roles. Although the substrate recognition mechanism of autophagy has been clarified in detail, that of GOMED is completely unknown. Here we report that GOMED degrades its substrate proteins selectively via optineurin (OPTN), as we found that the degradation of GOMED substrates is s`uppressed by the loss of OPTN. OPTN binds to K33 polyubiquitin-tagged proteins that have passed through the Golgi, which are then incorporated into GOMED structures for eventual degradation. In vivo, GOMED is known to be involved in the removal of mitochondria from erythrocytes, and in Optn-deficient mice, mitochondria are not degraded by GOMED, resulting in the appearance of erythrocytes containing mitochondria. These findings provide insight into the substrate recognition mechanism of GOMED.