2025-10-28 東京科学大学
Web要約 の発言:
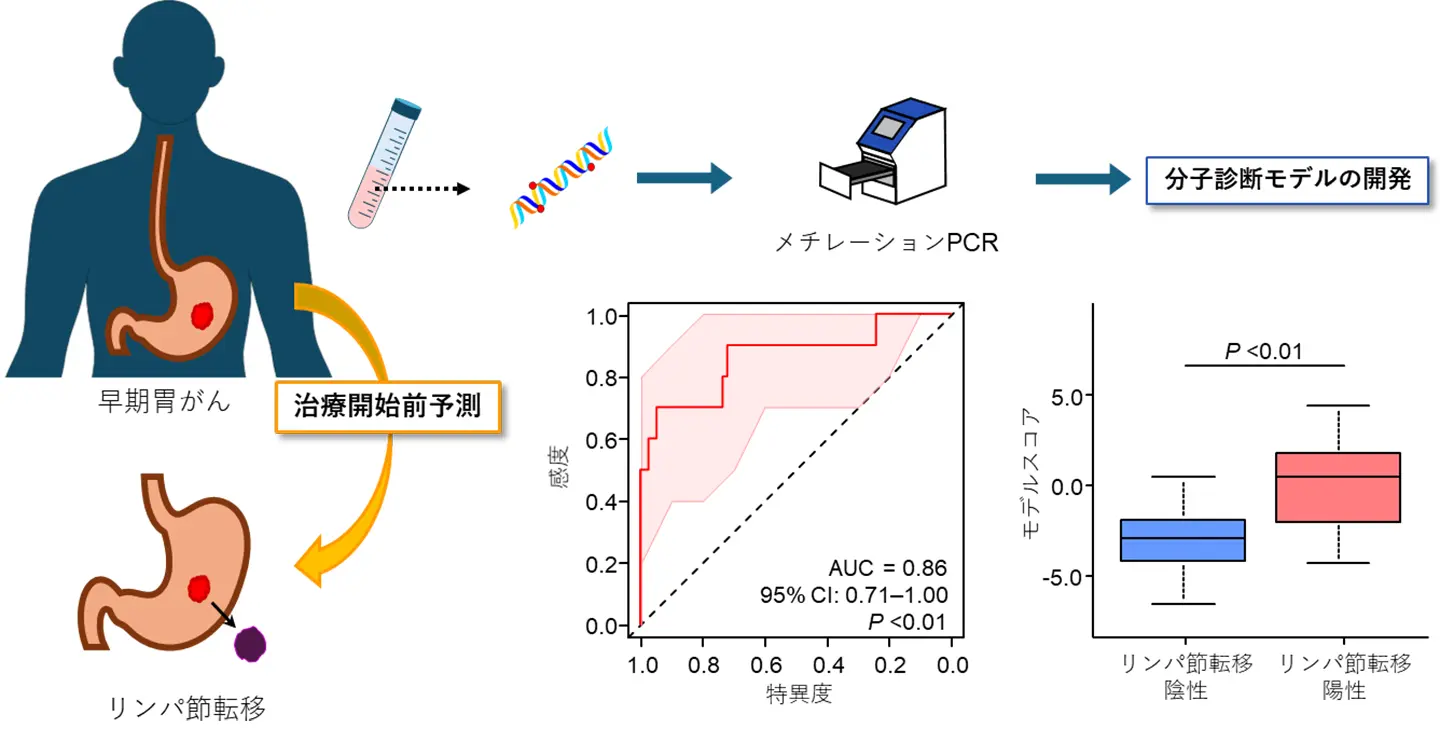
図1. 本研究成果の概要。治療開始前に採取した早期胃がん患者の血液検体からDNAを抽出し、メチレーションPCR法を使ってDNAメチル化レベルを測定し、早期胃がんのリンパ節転移リスクを治療開始前に予測するリキッドバイオプシー分子診断モデルを開発した。
<関連情報>
T1胃癌におけるリンパ節転移を同定するための細胞フリーDNAメチル化に基づく液体生検アッセイ Cell-Free DNA Methylation-Based Liquid Biopsy Assay to Identify Lymph Node Metastasis in T1 Gastric Cancer
Keisuke Okuno, Adwait Joshi, Shuichi Watanabe, Sakiko Oba, Kenta Yao, Toshiro Tanioka, Masanori Tokunaga, Daisuke Ban, Yusuke Kinugasa
United European Gastroenterology Journal Published: 17 October 2025
DOI:https://doi.org/10.1002/ueg2.70132
ABSTRACT
Background
Most T1 gastric cancer (GC) harbor lymph node metastasis (LNM) at a rate of < 20%; however, owing to the difficulty in accurately diagnosing LNM preoperatively, many patients with T1 GC undergo unnecessary invasive radical gastrectomy with lymphadenectomy. In the present study, we established an epigenetic liquid biopsy assay for the preoperative diagnosis of LNM in T1 GC.
Methods
A comprehensive biomarker discovery was performed by analyzing genome-wide DNA methylation profiling. We obtained 277 clinical specimens, including 177 surgical tissues and 100 pre-operative plasmas. DNA methylation biomarkers were trained and validated using quantitative methylation-specific polymerase chain reaction (qMSP) assays.
Results
We identified six novel differentially methylated regions, including at least two differentially methylated CpG probes (|Delta-beta| > 0.12 and p < 0.05) within 100 bp, through genome-wide biomarker discovery. A DNA methylation panel was generated using qMSP assays in clinical tissue specimens, with an area under the curve (AUC) of 0.80. This panel was validated in an independent clinical cohort, and a combined model, which integrated the DNA methylation model with preoperative computed tomography -based findings, was established through multivariate logistic regression analyses (AUC: 0.84). Finally, we translated this model into a liquid biopsy, and this cell-free DNA (cfDNA) methylation model exhibited robust performance for LNM identification in T1 GC (AUC: 0.86) and allowed 44% of patients to avoid unnecessary invasive operations, without missing any LNM-positive patients.
Conclusions
We have successfully developed a cfDNA methylation signature-based liquid biopsy diagnostic assay that allows for robust and less-invasive LNM detection in patients with T1 GC.
Key Summary
- Summarize the established knowledge on this subject
- Most T1 gastric cancers (GCs) harbor lymph node metastasis (LNM) at a rate of < 20%, and the 5-year survival rate of these patients has been reported to exceed 90%.
- Endoscopic resection for T1 GC is a function-preserving and less-invasive procedure; however, owing to the difficulty in accurately diagnosing LNM preoperatively, many patients undergo unnecessary invasive radical gastrectomy with lymphadenectomy.
- DNA methylation signatures are considered to be promising diagnostic biomarkers for LNM in T1 GC, and several studies have attempted to develop surgical tissue-based biomarkers for T1 GC, which have not yet been applied in clinical practice.
- There are no previous studies applying DNA methylation biomarkers for the detection of LNM in T1 GC to liquid biopsy assays.
- What are the significant and/or new findings of this study?
- Six novel DNA methylation biomarkers for LNM detection in T1 GC were identified by comprehensively analyzing genome-wide DNA methylation profiling.
- The combined model comprising six DNA methylation biomarkers and preoperative CT-based findings demonstrated a robust performance for the identification of LNM in T1 GC and was converted into a liquid biopsy assay.
- The novel cell-free DNA methylation-based liquid biopsy model allowed 44% of patients with T1 GC to avoid unnecessary invasive operations, without missing any LNM-positive patients.

