2025-12-11 ノースウェスタン大学
<関連情報>
- https://news.northwestern.edu/stories/2025/12/shape-shifting-cell-channel-reveals-new-target-for-precision-drugs
- https://www.nature.com/articles/s41467-025-66028-9
- https://www.nature.com/articles/s41586-020-2357-y
PANX1の浸透とメフロキンによる正の調節の構造的基盤 Structural basis of PANX1 permeation and positive modulation by mefloquine
Yangyang Li,Zheng Ruan,Junuk Lee,Ian J. Orozco,Edward Zhou,Juan Du & Wei Lü
Nature Communications Published:11 December 2025
DOI:https://doi.org/10.1038/s41467-025-66028-9
Abstract
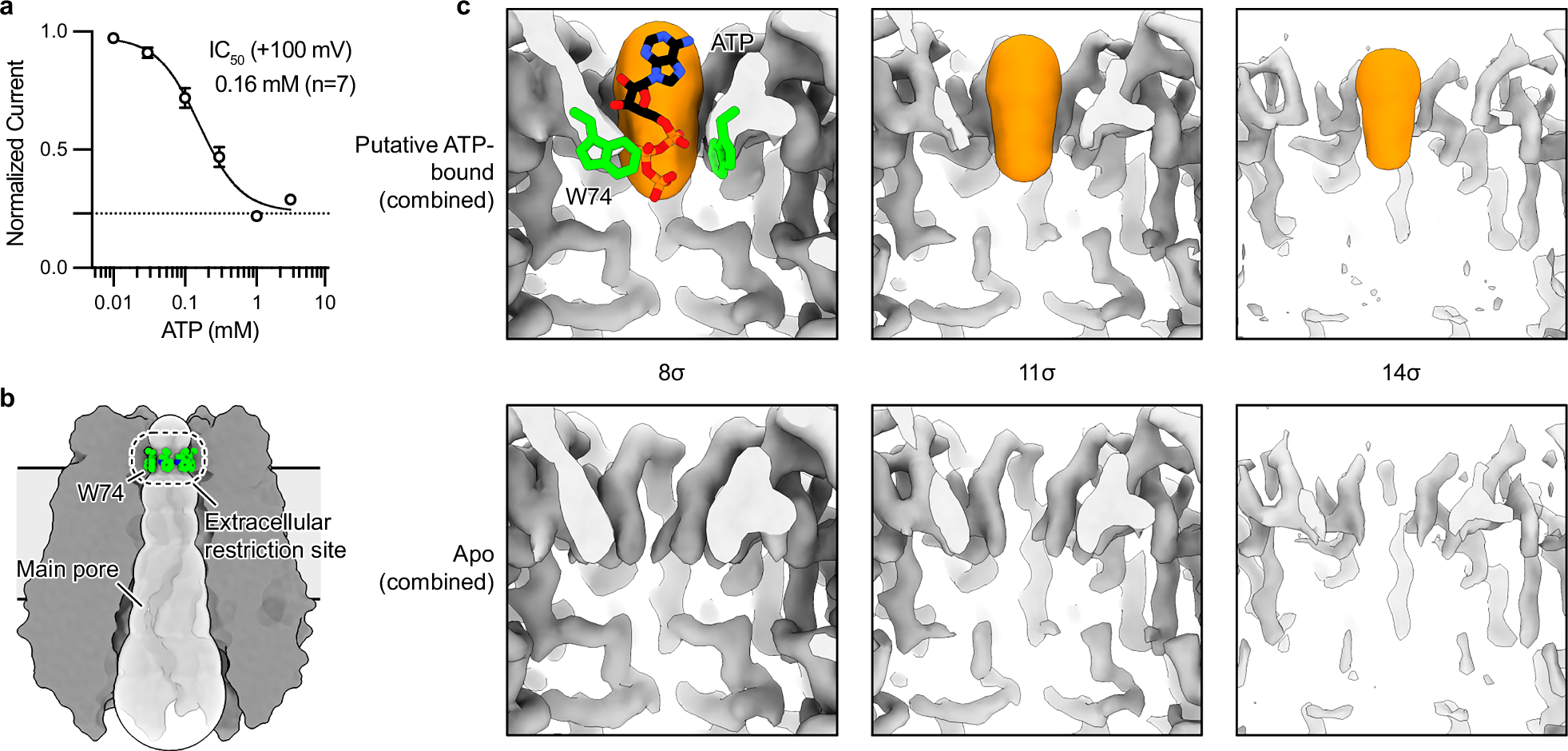
Purinergic signaling relies on ATP release through exocytosis and large-pore channels. Large-pore channels permeate both small anions like chloride and large signaling molecules like ATP, but how this broad cargo selectivity is structurally controlled remains elusive. Here we investigate PANX1, a prototypical large-pore channel, and uncover structural plasticity at the extracellular entrance formed by seven tryptophan (W74) residues. The W74 sidechains are flexible, sampling conformations that range from a constricted state permissive only to chloride to a dilated state compatible with ATP. These states are coupled to variable cation–π interactions between W74 and arginine 75 (R75), suggesting a mechanism for dynamic tuning of pore architecture and selective cargo permeation. We also identify mefloquine as a positive modulator of PANX1 that binds near the side tunnel to control ion flow through this pathway. Together, these findings define the structural principles underlying PANX1 permeation and modulation.
ヒトパネキシン1の構造はイオン経路とゲーティングのメカニズムを明らかにする Structures of human pannexin 1 reveal ion pathways and mechanism of gating
Zheng Ruan,Ian J. Orozco,Juan Du & Wei Lü
Nature Published:03 June 2020
DOI:https://doi.org/10.1038/s41586-020-2357-y
Abstract
Pannexin 1 (PANX1) is an ATP-permeable channel with critical roles in a variety of physiological functions such as blood pressure regulation1, apoptotic cell clearance2 and human oocyte development3. Here we present several structures of human PANX1 in a heptameric assembly at resolutions of up to 2.8 angström, including an apo state, a caspase-7-cleaved state and a carbenoxolone-bound state. We reveal a gating mechanism that involves two ion-conducting pathways. Under normal cellular conditions, the intracellular entry of the wide main pore is physically plugged by the C-terminal tail. Small anions are conducted through narrow tunnels in the intracellular domain. These tunnels connect to the main pore and are gated by a long linker between the N-terminal helix and the first transmembrane helix. During apoptosis, the C-terminal tail is cleaved by caspase, allowing the release of ATP through the main pore. We identified a carbenoxolone-binding site embraced by W74 in the extracellular entrance and a role for carbenoxolone as a channel blocker. We identified a gap-junction-like structure using a glycosylation-deficient mutant, N255A. Our studies provide a solid foundation for understanding the molecular mechanisms underlying the channel gating and inhibition of PANX1 and related large-pore channels.