2026-02-11 ノースウェスタン大学

Fluorescent micrographs showing increased neurite outgrowth from a human spinal cord organoid treated with fast-moving “dancing molecules” (left) compared to one treated with slow-moving molecules (right) containing the same bioactive signals.
<関連情報>
- https://news.northwestern.edu/stories/2026/02/paralysis-treatment-heals-lab-grown-human-spinal-cord-organoids
- https://www.science.org/doi/10.1126/science.abh3602
- https://www.nature.com/articles/s41551-025-01606-2
超分子運動を強化した生体活性スキャフォールドが脊髄損傷からの回復を促進 Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury
Z. Álvarez, A. N. Kolberg-Edelbrock, I. R. Sasselli, J. A. Ortega, […] , and S. I. Stupp
Science Published:11 Nov 2021
DOI:https://doi.org/10.1126/science.abh3602
Fibril motion improves peptide signaling
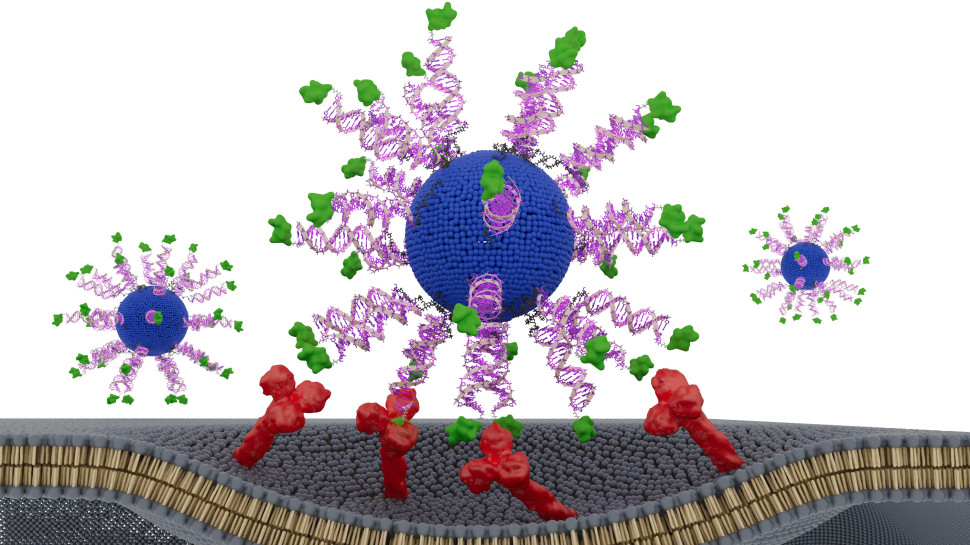
Artificial scaffolds that bear the peptide-signaling sequences of proteins for tissue regeneration often have limited effectiveness. Álvarez et al. synthesized supramolecular peptide fibril scaffolds bearing two peptide sequences that promote nerve regeneration, one that reduces glial scarring and another that promotes blood vessel formation (see the Perspective by Wojciechowski and Stevens). In a mouse model of paralyzing human spinal cord injury, mutations in a tetrapeptide domain outside of the signaling regions improved recovery by promoting intense supramolecular motion within the fibrils. The mutation with the most intense dynamics resulted in corticospinal axon regrowth and myelination, functional revascularization, and motor neuron survival. —PDS
Abstract
The signaling of cells by scaffolds of synthetic molecules that mimic proteins is known to be effective in the regeneration of tissues. Here, we describe peptide amphiphile supramolecular polymers containing two distinct signals and test them in a mouse model of severe spinal cord injury. One signal activates the transmembrane receptor β1-integrin and a second one activates the basic fibroblast growth factor 2 receptor. By mutating the peptide sequence of the amphiphilic monomers in nonbioactive domains, we intensified the motions of molecules within scaffold fibrils. This resulted in notable differences in vascular growth, axonal regeneration, myelination, survival of motor neurons, reduced gliosis, and functional recovery. We hypothesize that the signaling of cells by ensembles of molecules could be optimized by tuning their internal motions.
ヒト脊髄オルガノイドにおける損傷と治療 Injury and therapy in a human spinal cord organoid
Nozomu Takata,Zhiwei Li,Anna Metlushko,Feng Chen,Nicholas A. Sather,Xinyi Lin,Matthew J. Schipma,Oscar A. Carballo-Molina,Cassandre Jamroz,Madison E. Strong,Cara S. Smith,Yang Yang,Ching M. Wai,Neha Joshi,Jack Kolberg-Edelbrock,Kyle J. Gray,Suitu Wang,Liam C. Palmer & Samuel I. Stupp
Nature Biomedical Engineering Published:11 February 2026
DOI:https://doi.org/10.1038/s41551-025-01606-2
Abstract
Damage to the spinal cord can lead to irreversible paralysis and loss of sensory function, but translation of preclinical therapies remains elusive. We recently showed that bioactive supramolecular assemblies of peptide amphiphiles can reverse paralysis in an acute mouse model following severe spinal cord injury (SCI). Here we report the development of two human spinal cord organoid injury models to simulate SCI in vitro, a laceration of the organoid with a scalpel and a compressive contusion commonly used in preclinical models, both resulting in immediate neuronal death and the formation of glial scar-like tissue. Treatment of the injured organoids with the preclinical therapy suppressed the scar-like tissue and promoted significant axonal regeneration, as observed previously in vivo. With the inclusion of microglia into the spinal cord organoids, we demonstrate that the supramolecular nanomaterial reduced pro-inflammatory factors commonly associated with injury. The human spinal cord organoid models developed here could accelerate the discovery of therapies to treat SCI and possibly damage of other central nervous system tissues owing to trauma or disease.