2023-07-20 カリフォルニア大学サンタバーバラ校(UCSB)
◆この研究は、立体化学の制御において重要な一歩を踏み出し、将来的に学術と産業の両方でキラル化合物の合成と研究を促進することが期待されています。
<関連情報>
- https://www.news.ucsb.edu/2023/021133/toolbox-biocatalysts-improves-control-over-free-radicals
- https://www.nature.com/articles/s41929-023-00986-5
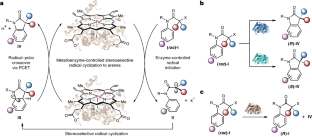
メタロレドックス生体触媒反応を利用した酵素制御によるアレーンへの立体選択的ラジカル環化反応 Enzyme-controlled stereoselective radical cyclization to arenes enabled by metalloredox biocatalysis
Wenzhen Fu,Natalia M. Neris,Yue Fu,Yunlong Zhao,Benjamin Krohn-Hansen,Peng Liu & Yang Yang
Nature Catalysis Published:20 July 2023
DOI:https://doi.org/10.1038/s41929-023-00986-5
Abstract
The effective induction of high levels of stereocontrol for free-radical-mediated transformations represents a notorious challenge in asymmetric catalysis. Herein, we describe a metalloredox biocatalysis strategy to repurpose natural cytochromes P450 to catalyse asymmetric radical cyclization to arenes through an unnatural electron transfer mechanism. Directed evolution afforded a series of engineered P450 aromatic radical cyclases with complementary selectivities: P450arc1 and P450arc2 facilitated enantioconvergent transformations of racemic substrates, giving rise to either enantiomer of the product with excellent total turnover numbers (up to 12,000). In addition to these enantioconvergent variants, another engineered radical cyclase, P450arc3, permitted efficient kinetic resolution of racemic chloride substrates (S factor = 18). Furthermore, computational studies revealed a proton-coupled electron transfer mechanism for the radical–polar crossover step, suggesting the potential role of the haem carboxylate as a base catalyst. Collectively, the excellent tunability of this metalloenzyme family provides an exciting platform for harnessing free radical intermediates for asymmetric catalysis.