2023-12-04 ラトガース大学
◆ラトガース大学主導の2つの研究チームは、別々のヒトとマウスのデータを使用して、これらの質問に「はい」と答える手がかりを見つけ、Nature CommunicationsおよびAmerican Journal of Human Geneticsに報告しました。これにより、体外受精(IVF)の成功率向上に向けた基本的なメカニズムの理解が可能になり、不妊治療の進化に寄与する可能性があります。
<関連情報>
- https://www.rutgers.edu/news/scientists-shed-light-mysteries-associated-infertility
- https://www.cell.com/ajhg/fulltext/S0002-9297(23)00397-X
- https://www.nature.com/articles/s41467-023-43288-x
着床前遺伝学的検査からの超低カバレッジ全ゲノムシーケンスを用いた胚異数性リスク変異の同定 Identifying risk variants for embryo aneuploidy using ultra-low coverage whole-genome sequencing from preimplantation genetic testing
Siqi Sun,Mansour Aboelenain,Daniel Ariad,Mary E. Haywood,Charles R. Wageman,Marlena Duke,Aishee Bag,Manuel Viotti,Mandy Katz-Jaffe,Rajiv C. McCoy,Karen Schindler,Jinchuan Xing
American Journal of Human Genetics Published:November 28, 2023
DOI:https://doi.org/10.1016/j.ajhg.2023.11.002
Summary
Aneuploidy frequently arises during human meiosis and is the primary cause of early miscarriage and in vitro fertilization (IVF) failure. Individuals undergoing IVF exhibit significant variability in aneuploidy rates, although the exact genetic causes of the variability in aneuploid egg production remain unclear. Preimplantation genetic testing for aneuploidy (PGT-A) using next-generation sequencing is a standard test for identifying and selecting IVF-derived euploid embryos. The wealth of embryo aneuploidy data and ultra-low coverage whole-genome sequencing (ulc-WGS) data from PGT-A have the potential to discover variants in parental genomes that are associated with aneuploidy risk in their embryos. Using ulc-WGS data from ∼10,000 PGT-A biopsies, we imputed genotype likelihoods of genetic variants in embryo genomes. We then used the imputed variants and embryo aneuploidy calls to perform a genome-wide association study of aneuploidy incidence. Finally, we carried out functional evaluation of the identified candidate gene in a mouse oocyte system. We identified one locus on chromosome 3 that is significantly associated with meiotic aneuploidy risk. One candidate gene, CCDC66, encompassed by this locus, is involved in chromosome segregation during meiosis. Using mouse oocytes, we showed that CCDC66 regulates meiotic progression and chromosome segregation fidelity, especially in older mice. Our work extended the research utility of PGT-A ulc-WGS data by allowing robust association testing and improved the understanding of the genetic contribution to maternal meiotic aneuploidy risk. Importantly, we introduce a generalizable method that has potential to be leveraged for similar association studies that use ulc-WGS data.
Graphical abstract

マウスにおける卵子の減数分裂中期体キャップは発生能力に必要である。 An oocyte meiotic midbody cap is required for developmental competence in mice
Gyu Ik Jung,Daniela Londoño-Vásquez,Sungjin Park,Ahna R. Skop,Ahmed Z. Balboula & Karen Schindler
Nature Communications Published:16 November 2023
DOI:https://doi.org/10.1038/s41467-023-43288-x
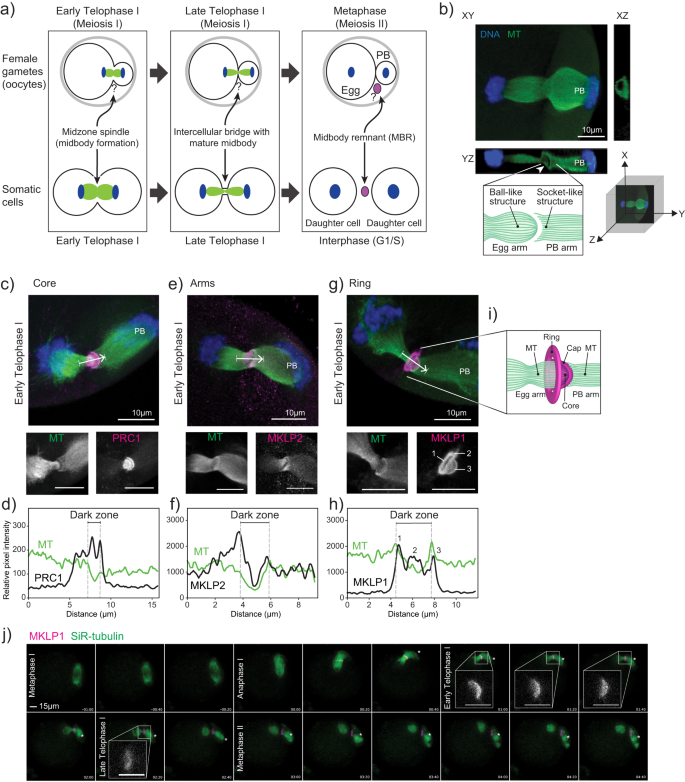
Abstract
Embryo development depends upon maternally derived materials. Mammalian oocytes undergo extreme asymmetric cytokinesis events, producing one large egg and two small polar bodies. During cytokinesis in somatic cells, the midbody and subsequent assembly of the midbody remnant, a signaling organelle containing RNAs, transcription factors and translation machinery, is thought to influence cellular function or fate. The role of the midbody and midbody remnant in gametes, in particular, oocytes, remains unclear. Here, we examined the formation and function of meiotic midbodies (mMB) and mMB remnants using mouse oocytes and demonstrate that mMBs have a specialized cap structure that is orientated toward polar bodies. We show that that mMBs are translationally active, and that mMB caps are required to retain nascent proteins in eggs. We propose that this specialized mMB cap maintains genetic factors in eggs allowing for full developmental competency.