2024-02-02 バーミンガム大学
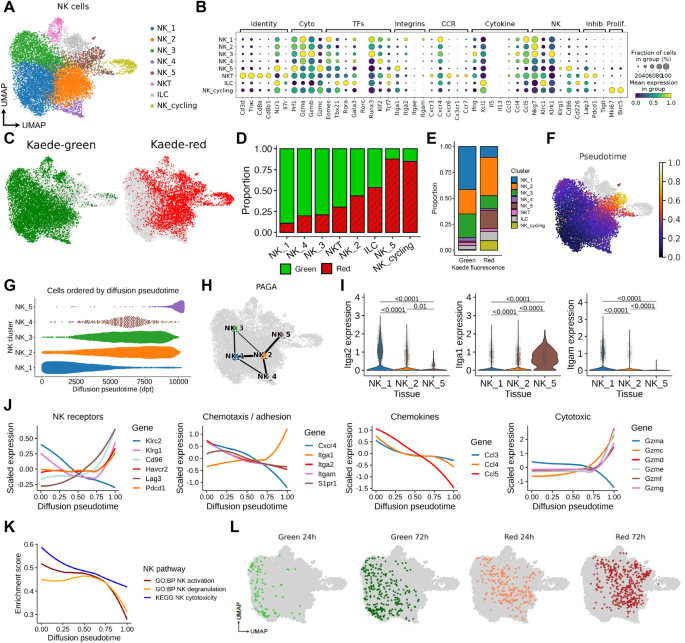
◆マウスモデルで育てられた腫瘍細胞を使用し、研究チームはNK細胞が固形腫瘍に入ると休眠状態になり、免疫反応を促進するために使用される主要な効果メカニズムの生成が失われることを確立した。
◆さらに、人間の結腸がんから採取された細胞を含む追加の研究で、自然キラー細胞の機能喪失が人間でも起こることが確認された。これは固形腫瘍がんを扱うための新しい治療法の可能性を示唆している。
<関連情報>
- https://www.birmingham.ac.uk/news/2024/immune-cells-lose-killer-instinct-in-cancerous-tumours-but-functionality-can-be-re-awakened
- https://www.nature.com/articles/s41467-024-44789-z#Abs1
- https://www.nature.com/articles/s41467-024-44787-1
腫瘍侵入後のナチュラルキラー細胞の急速な機能障害が抗腫瘍免疫を制限する Rapid functional impairment of natural killer cells following tumor entry limits anti-tumor immunity
Isaac Dean,Colin Y. C. Lee,Zewen K. Tuong,Zhi Li,Christopher A. Tibbitt,Claire Willis,Fabrina Gaspal,Bethany C. Kennedy,Veronika Matei-Rascu,Rémi Fiancette,Caroline Nordenvall,Ulrik Lindforss,Syed Murtuza Baker,Christian Stockmann,Veronika Sexl,Scott A. Hammond,Simon J. Dovedi,Jenny Mjösberg,Matthew R. Hepworth,Gianluca Carlesso,Menna R. Clatworthy & David R. Withers
Nature Communications Published:24 January 2024
DOI:https://doi.org/10.1038/s41467-024-44789-z
Abstract
Immune cell dysfunction within the tumor microenvironment (TME) undermines the control of cancer progression. Established tumors contain phenotypically distinct, tumor-specific natural killer (NK) cells; however, the temporal dynamics, mechanistic underpinning and functional significance of the NK cell compartment remains incompletely understood. Here, we use photo-labeling, combined with longitudinal transcriptomic and cellular analyses, to interrogate the fate of intratumoral NK cells. We reveal that NK cells rapidly lose effector functions and adopt a distinct phenotypic state with features associated with tissue residency. NK cell depletion from established tumors did not alter tumor growth, indicating that intratumoral NK cells cease to actively contribute to anti-tumor responses. IL-15 administration prevented loss of function and improved tumor control, generating intratumoral NK cells with both tissue-residency characteristics and enhanced effector function. Collectively, our data reveals the fate of NK cells after recruitment into tumors and provides insight into how their function may be revived.
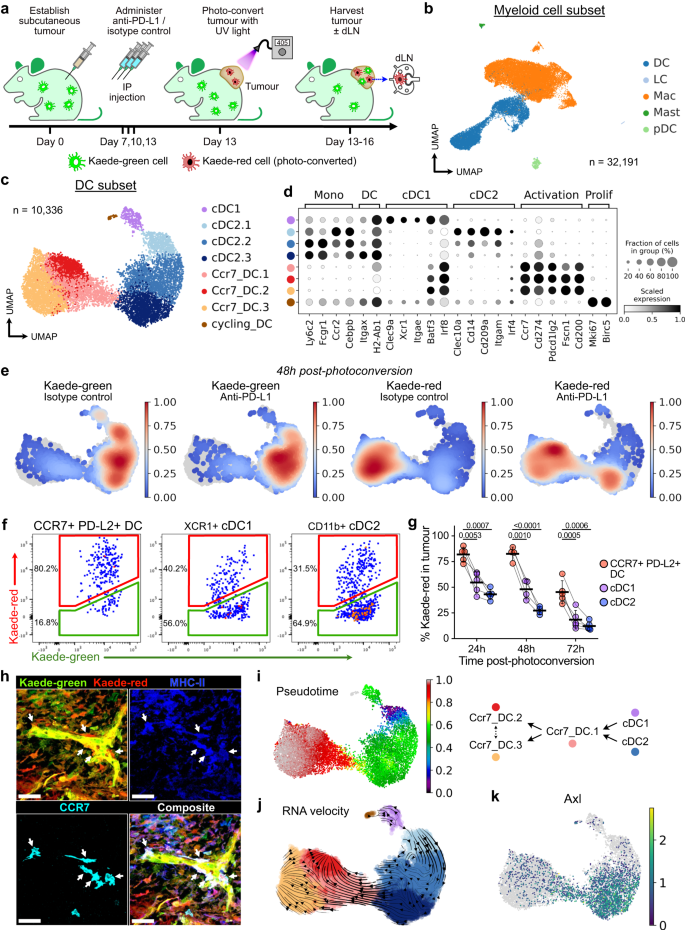
腫瘍に保持された活性化CCR7+樹状細胞は不均一であり、局所的な抗腫瘍細胞溶解活性を制御している。 Tumour-retained activated CCR7+ dendritic cells are heterogeneous and regulate local anti-tumour cytolytic activity
Colin Y. C. Lee, Bethany C. Kennedy, Nathan Richoz, Isaac Dean, Zewen K. Tuong, Fabrina Gaspal, Zhi Li, Claire Willis, Tetsuo Hasegawa, Sarah K. Whiteside, David A. Posner, Gianluca Carlesso, Scott A. Hammond, Simon J. Dovedi, Rahul Roychoudhuri, David R. Withers & Menna R. Clatworthy
Nature Communications volume 15, Article number: 682 (2024) Cite this article
Abstract
Tumour dendritic cells (DCs) internalise antigen and upregulate CCR7, which directs their migration to tumour-draining lymph nodes (dLN). CCR7 expression is coupled to an activation programme enriched in regulatory molecule expression, including PD-L1. However, the spatio-temporal dynamics of CCR7+ DCs in anti-tumour immune responses remain unclear. Here, we use photoconvertible mice to precisely track DC migration. We report that CCR7+ DCs are the dominant DC population that migrate to the dLN, but a subset remains tumour-resident despite CCR7 expression. These tumour-retained CCR7+ DCs are phenotypically and transcriptionally distinct from their dLN counterparts and heterogeneous. Moreover, they progressively downregulate the expression of antigen presentation and pro-inflammatory transcripts with more prolonged tumour dwell-time. Tumour-residing CCR7+ DCs co-localise with PD-1+CD8+ T cells in human and murine solid tumours, and following anti-PD-L1 treatment, upregulate stimulatory molecules including OX40L, thereby augmenting anti-tumour cytolytic activity. Altogether, these data uncover previously unappreciated heterogeneity in CCR7+ DCs that may underpin a variable capacity to support intratumoural cytotoxic T cells.