2024-02-21 カリフォルニア大学サンタバーバラ校(UCSB)
◆彼らは、これらの化合物の抗微生物活性に注目し、後の研究でこれに取り組みました。今日に至り、彼らは広範な細菌感染に対する約束のある新しい抗生物質の基礎を持っています。また、現行世代の第一選択抗生物質を無効にしている恐れのある耐性を回避できる可能性もあります。
<関連情報>
- https://news.ucsb.edu/2024/021365/researchers-develop-molecules-new-class-antibiotics-can-overcome-drug-resistant
- https://www.science.org/doi/10.1126/scitranslmed.adi7558
- https://pubs.acs.org/doi/10.1021/acs.jmedchem.3c01329
- https://pubs.rsc.org/en/content/articlelanding/2023/cc/d3cc02861e
膿瘍菌に有効な抗マイコバクテリア結合オリゴ電解質 An anti-mycobacterial conjugated oligoelectrolyte effective against Mycobacterium abscessus
KAIXI ZHANG , JAKKARIN LIMWONGYUT , ALEX S. MORELAND , SAMUEL CHAN JUN WEI , […], AND GUILLERMO C. BAZAN
Science Translational Medicine Published:21 Feb 2024
DOI:https://doi.org/10.1126/scitranslmed.adi7558
Editor’s summary
Nontuberculous mycobacteria cause substantial infective burden and can be difficult to treat using current regimens. Zhang et al. show that a conjugated oligoelectrolyte, whose structure is unrelated to current antibiotics, was bactericidal against both replicating and nonreplicating M. abscessus. The compound was also effective against intracellular infection. Treatment of a mouse lung infection model was efficacious with no signs of toxicity and low resistance development, illustrating the molecule’s therapeutic potential. —Catherine Charneski
Abstract
Infections caused by nontuberculous mycobacteria have increased more than 50% in the past two decades and more than doubled in the elderly population. Mycobacterium abscessus (Mab), one of the most prevalent of these rapidly growing species, is intrinsically resistant to numerous antibiotics. Current standard-of-care treatments are not satisfactory, with high failure rate and notable adverse effects. We report here a potent anti-Mab compound from the flexible molecular framework afforded by conjugated oligoelectrolytes (COEs). A screen of structurally diverse, noncytotoxic COEs identified a lead compound, COE-PNH2, which was bactericidal against replicating, nonreplicating persisters and intracellular Mab.COE-PNH2 had low propensity for resistance development, with a frequency of resistance below 1.25 × 10-9 and showed no detectable resistance upon serial passaging. Mechanism of action studies were in line with COE-PNH2 affecting the physical and functional integrity of the bacterial envelope and disrupting the mycomembrane and associated essential bioenergetic pathways. Moreover, COE-PNH2 was well-tolerated and efficacious in a mouse model of Mab lung infection. This study highlights desirable in vitro and in vivo potency and safety index of this COE structure, which represents a promising anti-mycobacterial to tackle an unmet medical need.
緑膿菌バイオフィルムに対する抗菌活性を有するアミジン系カチオン性共役オリゴ電解質 Amidine-Based Cationic Conjugated Oligoelectrolytes with Antimicrobial Activity against Pseudomonas aeruginosa Biofilms
Jakkarin Limwongyut, Alex S. Moreland, Kaixi Zhang, Malik Raynor, Supaksorn Chattagul, Timothy A. Fitzgerald, Yoann Le Breton, Daniel V. Zurawski, and Guillermo C. Bazan
Journal of Medicinal Chemistry Published:October 5, 2023
DOI:https://doi.org/10.1021/acs.jmedchem.3c01329
Abstract

Pseudomonas aeruginosa is an opportunistic Gram-negative bacterium that can cause high-morbidity infections. Due to its robust, flexible genome and ability to form biofilms, it can evade and rapidly develop resistance to antibiotics. Cationic conjugated oligoelectrolytes (COEs) have emerged as a promising class of antimicrobials. Herein, we report a series of amidine-containing COEs with high selectivity for bacteria. From this series, we identified 1b as the most active compound against P. aeruginosa (minimum inhibitory concentration (MIC) = 2 μg/mL) with low cytotoxicity (IC50 (HepG2) = 1024 μg/mL). The activity of 1b was not affected by known drug-resistant phenotypes of 100 diverse P. aeruginosa isolates. Moreover, 1b is bactericidal with a low propensity for P. aeruginosa to develop resistance. Furthermore, 1b is also able to inhibit biofilm formation at subinhibitory concentrations and kills P. aeruginosa in established biofilms. The in vivo efficacy of 1b was demonstrated in biofilm-associated murine wound infection models.
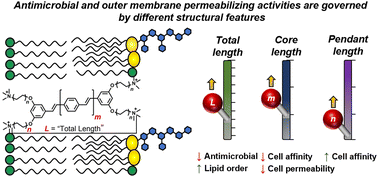
膜貫通型共役系オリゴ電解質の構造制御により外膜透過活性と抗菌活性が分離する Structural modulation of membrane-intercalating conjugated oligoelectrolytes decouples outer membrane permeabilizing and antimicrobial activities
Alex S. Moreland,Jakkarin Limwongyut,Samuel J. Holton and Guillermo C. Bazan
Chemical Communications Published:04 Sep 2023
DOI:https://doi.org/10.1039/D3CC02861E
Abstract
We report a series of membrane-intercalating conjugated oligoelectrolytes (MICOEs) to probe how structural features impact bacterial membrane integrity and antibiotic activity. Minimum inhibitory concentrations (MICs) and outer membrane (OM) permeability correlated to different structural parameters suggesting that the antimicrobial mechanism is not related to OM permeabilization. However, lipid order parameters and MICs correlated to the same structural feature suggesting a possible link.