2024-05-21 シンガポール国立大学(NUS)
<関連情報>
- https://news.nus.edu.sg/muscle-cells-natural-defences-against-cancer-using-magnetic-pulses/
- https://www.mdpi.com/2073-4409/13/5/460
磁気刺激された筋肉から分泌されるセクレトームが抗がん作用を示す HTRA1の作用を強調する新しい前処理法 Secretome from Magnetically Stimulated Muscle Exhibits Anticancer Potency: Novel Preconditioning Methodology Highlighting HTRA1 Action
Yee Kit Tai,Jan Nikolas Iversen,Karen Ka Wing Chan,Charlene Hui Hua Fong,Rafhanah Banu Abdul Razar,Sharanya Ramanan,Lye Yee Jasmine Yap,Jocelyn Naixin Yin,Shi Jie Toh,Craig Jun Kit Wong,Pei Fern Angele Koh,Ruby Yun Ju Huang andAlfredo Franco-Obregón
Cells Published: 5 March 2024
DOI:https://doi.org/10.3390/cells13050460
Abstract
Briefly (10 min) exposing C2C12 myotubes to low amplitude (1.5 mT) pulsed electromagnetic fields (PEMFs) generated a conditioned media (pCM) that was capable of mitigating breast cancer cell growth, migration, and invasiveness in vitro, whereas the conditioned media harvested from unexposed myotubes, representing constitutively released secretome (cCM), was less effective. Administering pCM to breast cancer microtumors engrafted onto the chorioallantoic membrane of chicken eggs reduced tumor volume and vascularity. Blood serum collected from PEMF-exposed or exercised mice allayed breast cancer cell growth, migration, and invasiveness. A secretome preconditioning methodology is presented that accentuates the graded anticancer potencies of both the cCM and pCM harvested from myotubes, demonstrating an adaptive response to pCM administered during early myogenesis that emulated secretome-based exercise adaptations observed in vivo. HTRA1 was shown to be upregulated in pCM and was demonstrated to be necessary and sufficient for the anticancer potency of the pCM; recombinant HTRA1 added to basal media recapitulated the anticancer effects of pCM and antibody-based absorption of HTRA1 from pCM precluded its anticancer effects. Brief and non-invasive PEMF stimulation may represent a method to commandeer the secretome response of muscle, both in vitro and in vivo, for clinical exploitation in breast and other cancers.
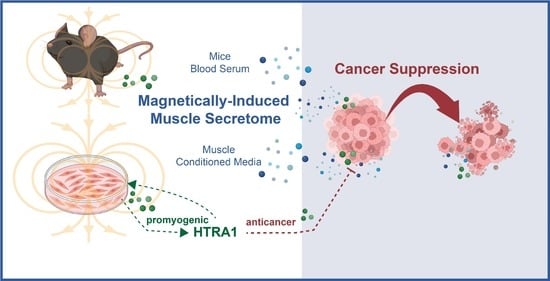
Graphical Abstract