2025-03-11 北海道大学
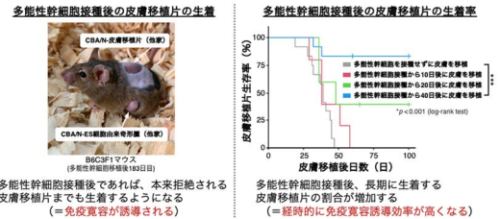
北海道大学遺伝子病制御研究所の清野研一郎教授らの研究チームは、多能性幹細胞(iPS細胞やES細胞)が移植免疫寛容を誘導する新たな機能を発見し、そのメカニズムを解明しました。 従来、他家移植では免疫拒絶反応を防ぐために免疫抑制剤の投与が必要とされていましたが、今回の研究では、特定の条件下で多能性幹細胞を他者のマウスに接種するだけで、免疫寛容が誘導されることが明らかになりました。この現象には、免疫制御性細胞である制御性T細胞(Treg)の増加が関与しており、Tregを除去すると免疫寛容が誘導されなくなることが確認されました。この成果は、多能性幹細胞の基礎生物学的理解を深めるとともに、再生医療における新たな免疫拒絶反応抑制法の開発につながる可能性があります。
<関連情報>
- https://www.hokudai.ac.jp/news/2025/03/post-1816.html
- https://www.hokudai.ac.jp/news/pdf/250311_pr.pdf
- https://www.pnas.org/doi/10.1073/pnas.2413398122
免疫抑制なしに同種宿主に移植されたiPSCは二次移植片に対してドナー特異的寛容を誘導する iPSCs engrafted in allogeneic hosts without immunosuppression induce donor-specific tolerance to secondary allografts
Tomoki Kamatani, Reiko Kimura, Satoshi Ikeda, +1 , and Ken-ichiro Seino
Proceedings of the National Academy of Sciences Published:March 12, 2025
DOI:https://doi.org/10.1073/pnas.2413398122
Significance
The immunological properties of induced pluripotent stem cells (iPSCs) during allogeneic transplantation have not yet been fully elucidated. In this study, we found that iPSCs exhibit immunologically unique behavior in major histocompatibility complex (MHC)-compatible/minor antigen-mismatched allogeneic combinations. When injected subcutaneously, iPSCs were not rejected and, 40 d later, induced immune tolerance in secondary transplanted grafts in a donor-specific manner. Transforming growth factor (TGF)-β2 expression and Treg behavior was associated with this phenomenon. The results of this study provide insights into the relationship between pluripotent stem cells and immune tolerance, offering crucial insights into the advancement of regenerative medicine using iPSCs.
Abstract
Currently, most cell or tissue transplantations using induced pluripotent stem cells (iPSCs) are anticipated to involve allogeneic iPSCs. However, the immunological properties of iPSCs in an allogeneic setting are not well understood. We previously established a mouse transplantation model of MHC-compatible/minor antigen-mismatched combinations, assuming a hypoimmunogenic iPSC-setting. Here, we found that iPSCs subcutaneously inoculated into MHC-compatible allogeneic host mice resisted rejection and formed teratomas without immunosuppressant administration. Notably, when skin grafts were transplanted onto hosts more than 40 d after the initial iPSCs inoculation, only the skin of the same strain as the initial iPSCs was engrafted. Therefore, donor-specific immune tolerance was induced by a single iPSC inoculation. Diverse analyses, including single-cell RNA-sequencing after transplantation, revealed an increase in regulatory T cell (Treg) population, particularly CD25+ CD103+ effector Tregs within the teratoma and skin grafts. The removal of CD25+ or Foxp3+ cells suppressed the increase in effector Tregs and disrupted graft acceptance, indicating the importance of these cells in the establishment of immune tolerance. Within the teratoma, we observed an increase in TGF-β2 levels, suggesting an association with the increase in effector Tregs. Our results provide important insights for future applications of allogeneic iPSC-based cell or tissue transplantation.