2025-03-12 兵庫県立大学
<関連情報>
- https://www.u-hyogo.ac.jp/news/pressrelease/20250312press.html
- https://www.u-hyogo.ac.jp/20250312press.pdf
- https://www.nature.com/articles/s42004-025-01440-2
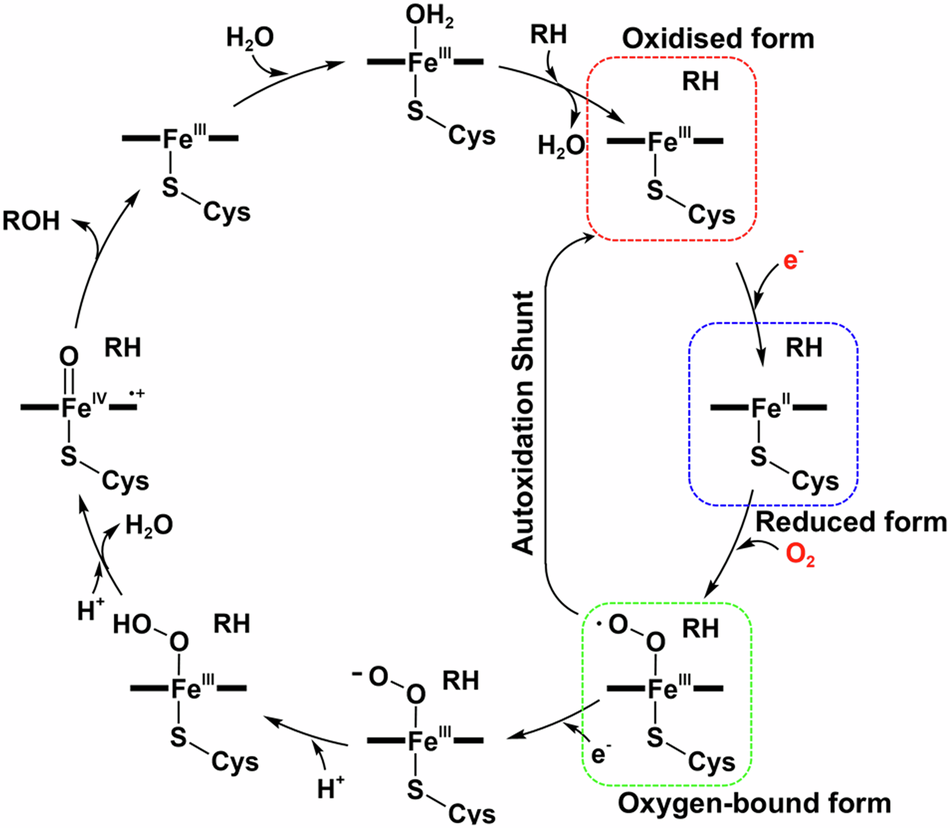
シトクロムP450BM3による非ネイティブ基質酸化反応における触媒サイクルのダイナミクスをXFEL結晶構造解析で解明 XFEL crystallography reveals catalytic cycle dynamics during non-native substrate oxidation by cytochrome P450BM3
Satoshi Nagao,Wako Kuwano,Takehiko Tosha,Keitaro Yamashita,Joshua Kyle Stanfield,Chie Kasai,Shinya Ariyasu,Kunio Hirata,Go Ueno,Hironori Murakami,Hideo Ago,Masaki Yamamoto,Osami Shoji,Hiroshi Sugimoto & Minoru Kubo
Communications Chemistry Published:12 March 2025
DOI:https://doi.org/10.1038/s42004-025-01440-2

Abstract
Cytochrome P450s are haem-containing enzymes, catalysing the regio- and stereospecific oxidation of non-activated hydrocarbons. Among these, the bacterial P450BM3 is a promising biocatalyst due to its high enzymatic activity. Given the significant conformational flexibility of this enzyme, understanding protein-substrate interactions and associated structural dynamics are crucial for designing P450BM3-based biocatalysts. Herein, employing an X-ray free electron laser in combination with freeze-trap crystallography and spectroscopy techniques, we captured the intact structures of engineered P450BM3s in the initial stages of catalysis during styrene epoxidation, in the presence of a decoy molecule. We found that the iron reduction significantly altered the active-site orientation of styrene, driven by structural changes in surrounding helices and hydrogen-bonding networks. Oxygen binding to iron further stabilised its productive orientation, providing a molecular basis for the experimentally observed enzyme kinetics and enantioselectivities. This study reveals the substrate dynamics of a P450 enzyme, showcasing how changes in haem chemistry affect enzyme structure and substrate orientation.