2025-03-18 京都大学
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-03-18
- https://www.kyoto-u.ac.jp/sites/default/files/2025-03/web_2503_Hayashi-72df176da025e0b11f16439d3a891092.pdf
- https://www.nature.com/articles/s41467-025-57456-8
CPC-シェルテリン-BTR軸が有糸分裂期のテロメア脱保護を制御する A CPC-shelterin-BTR axis regulates mitotic telomere deprotection
Diana Romero-Zamora,Samuel Rogers,Ronnie Ren Jie Low,Scott G. Page,Blake J. E. Lane,Shunya Kosaka,Andrew B. Robinson,Lucy French,Noa Lamm,Fuyuki Ishikawa,Makoto T. Hayashi & Anthony J. Cesare
Nature Communications Published:17 March 2025
DOI:https://doi.org/10.1038/s41467-025-57456-8
Abstract
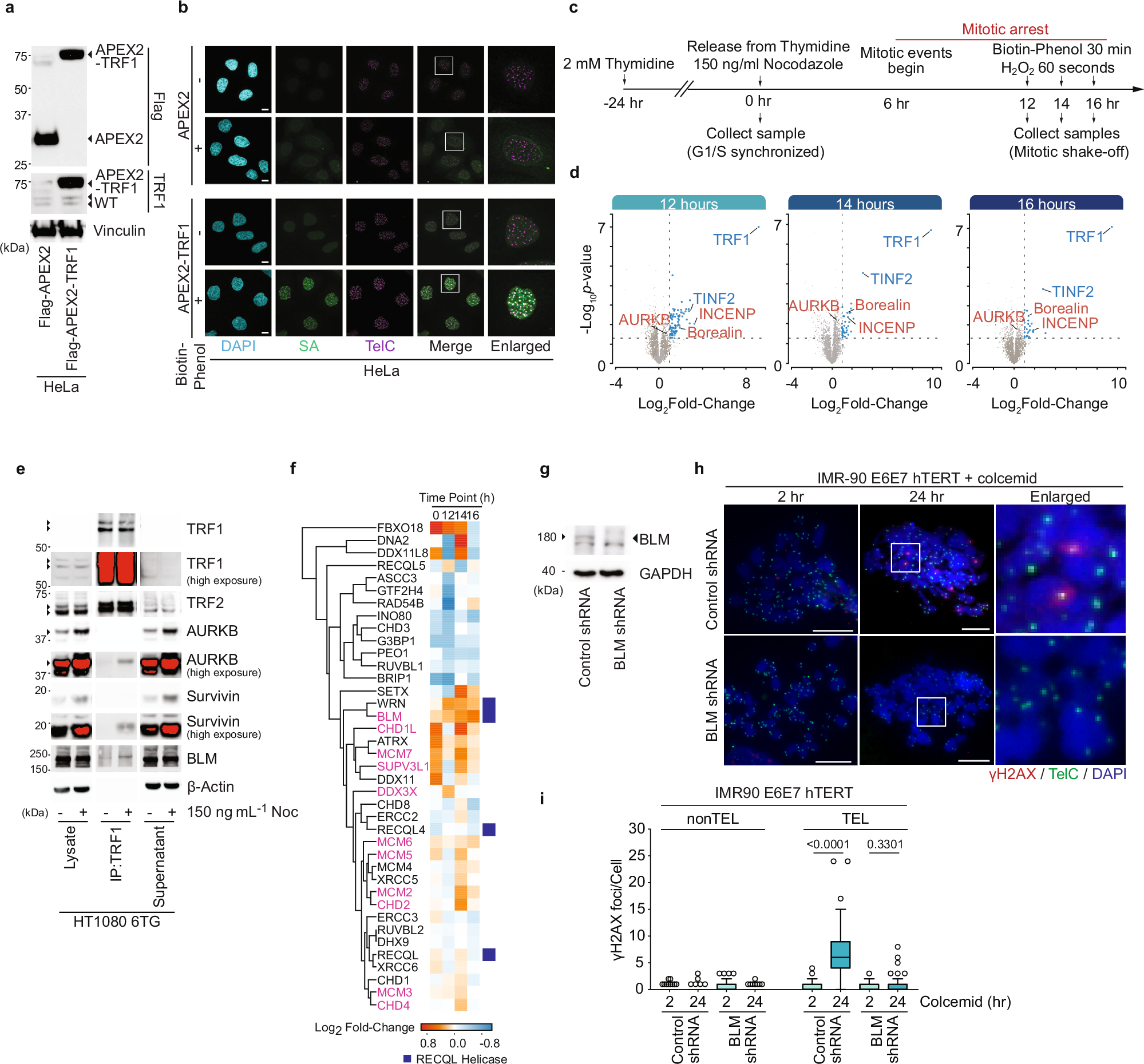
Telomeres prevent ATM activation by sequestering chromosome termini within telomere loops (t-loops). Mitotic arrest promotes telomere linearity and a localized ATM-dependent telomere DNA damage response (DDR) through an unknown mechanism. Using unbiased interactomics, biochemical screening, molecular biology, and super-resolution imaging, we found that mitotic arrest-dependent (MAD) telomere deprotection requires the combined activities of the Chromosome passenger complex (CPC) on shelterin, and the BLM-TOP3A-RMI1/2 (BTR) complex on t-loops. During mitotic arrest, the CPC component Aurora Kinase B (AURKB) phosphorylated both the TRF1 hinge and TRF2 basic domains. Phosphorylation of the TRF1 hinge domain enhances CPC and TRF1 interaction through the CPC Survivin subunit. Meanwhile, phosphorylation of the TRF2 basic domain promotes telomere linearity, activates a telomere DDR dependent on BTR-mediated double Holliday junction dissolution, and leads to mitotic death. We identify that the TRF2 basic domain functions in mitosis-specific telomere protection and reveal a regulatory role for TRF1 in controlling a physiological ATM-dependent telomere DDR. The data demonstrate that MAD telomere deprotection is a sophisticated active mechanism that exposes telomere ends to signal mitotic stress.