2025-07-15 国立がん研究センター,名古屋大学,京都大学,大阪大学,理化学研究所,産業技術総合研究所
<関連情報>
- https://www.ncc.go.jp/jp/information/pr_release/2025/0715/index.html
- https://www.ncc.go.jp/jp/information/pr_release/2025/0715/20250715.pdf
- https://www.nature.com/articles/s41586-025-09249-8
樹状細胞の移動を介した微生物叢主導型抗腫瘍免疫 Microbiota-driven antitumour immunity mediated by dendritic cell migration
Nina Yi-Tzu Lin,Shota Fukuoka,Shohei Koyama,Daisuke Motooka,Dieter M. Tourlousse,Yuko Shigeno,Yuki Matsumoto,Hiroyuki Yamano,Kazutoshi Murotomi,Hideyuki Tamaki,Takuma Irie,Eri Sugiyama,Shogo Kumagai,Kota Itahashi,Tokiyoshi Tanegashima,Kaori Fujimaki,Sachiko Ito,Mariko Shindo,Takahiro Tsuji,Hiroaki Wake,Keisuke Watanabe,Yuka Maeda,Tomohiro Enokida,Makoto Tahara,… Hiroyoshi Nishikawa
Nature Published:14 July 2025
DOI:https://doi.org/10.1038/s41586-025-09249-8
Abstract
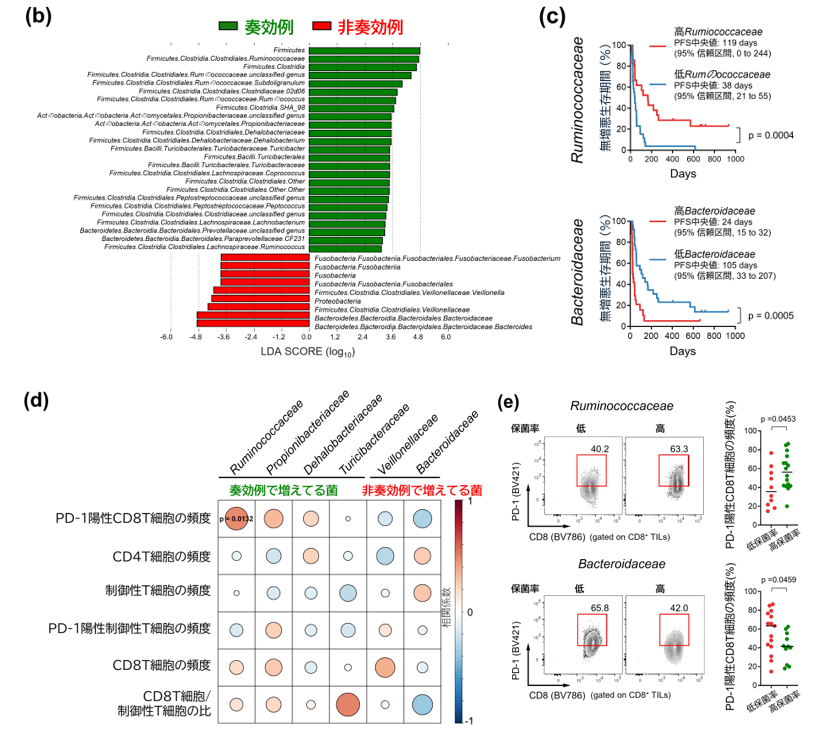
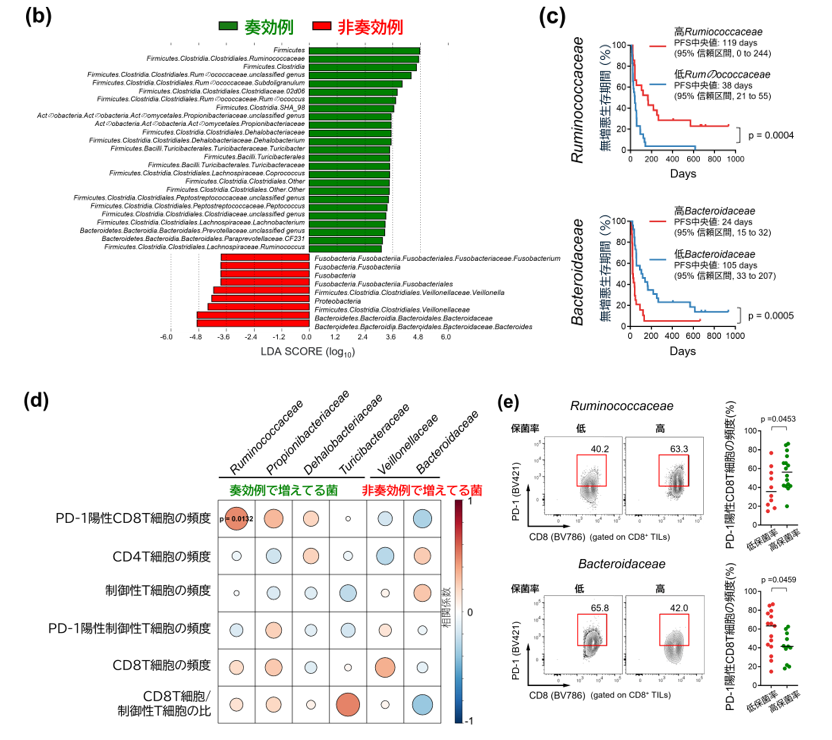
Gut microbiota influence the antitumour efficacy of immune checkpoint blockade1,2,3,4,5,6, but the mechanisms of action have not been fully elucidated. Here, we show that a new strain of the bacterial genus Hominenteromicrobium (designated YB328) isolated from the faeces of patients who responded to programmed cell death 1 (PD-1) blockade augmented antitumour responses in mice. YB328 activated tumour-specific CD8+ T cells through the stimulation of CD103+CD11b– conventional dendritic cells (cDCs), which, following exposure in the gut, migrated to the tumour microenvironment. Mice showed improved antitumour efficacy of PD-1 blockade when treated with faecal transplants from non-responder patients supplemented with YB238. This result suggests that YB328 could function in a dominant manner. YB328-activated CD103+CD11b– cDCs showed prolonged engagement with tumour-specific CD8+ T cells and promoted PD-1 expression in these cells. Moreover, YB238-augmented antitumour efficacy of PD-1 blockade treatment was observed in multiple mouse models of cancer. Patients with elevated YB328 abundance had increased infiltration of CD103+CD11b– cDCs in tumours and had a favourable response to PD-1 blockade therapy in various cancer types. We propose that gut microbiota enhance antitumour immunity by accelerating the maturation and migration of CD103+CD11b– cDCs to increase the number of CD8+ T cells that respond to diverse tumour antigens.