2025-07-16 マックス・プランク研究所
<関連情報>
- https://www.mpg.de/25066603/two-related-proteins-control-development-of-the-heart
- https://www.sciencedirect.com/science/article/pii/S1534580725003685
Rbpms2は心筋細胞特異的Rbpms欠損マウスにおける主要な心臓欠損を予防する Rbpms2 prevents major cardiac defects in cardiomyocyte-specific Rbpms-deficient mice
Shan Lin, Christoph Dieterich, Thiago Britto-Borges, Stefan Günther, Silke Kreher, Yvonne Eibach, Carsten Kuenne, Andre Schneider, Thomas Braun
Developmental Cell Available online: 1 July 2025
DOI:https://doi.org/10.1016/j.devcel.2025.06.013

Highlights
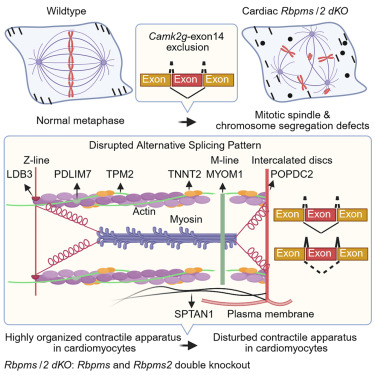
- RBPMS/2 concomitantly regulate alternative splicing in cardiomyocytes
- RBPMS/2 prevent formation of aberrant sarcomeres and nuclear defects
- Disbalance of Camk2g isoforms causes spindle and chromosome segregation defects
- Overexpression of Rbpmsa induces cardiomyocyte-specific alternative splicing
Summary
Cell-type-specific splicing depends on RNA-binding splicing factors. Several important splicing factors were identified in cardiomyocytes, including members of the RNA-binding proteins with multiple splicing (RBPMS) family, but their role during heart development has not been fully characterized. Here, we demonstrate that the function of RBPMS overlaps with the closely related paralog RBPMS2. Rbpms-deficient cardiomyocytes exhibit a higher degree of binucleation at birth, but this does not affect heart function in mice substantially until late adulthood. In contrast, Rbpms/Rbpms2 (Rbpms/2) compound mutants show pronounced disruption of the splicing network in embryonic cardiomyocytes, which leads to the formation of defective nuclei and disruption of sarcomere structures, eventually resulting in embryonic lethality. We demonstrate that mitotic defects in embryonic Rbpms/2-deficient cardiomyoctes are caused by the disbalance of nuclear and cytoplasmic calcium (Ca2+)/CaM-dependent protein kinase II gamma (Camk2g) isoforms. Overexpression of the Rbpmsa isoform partially rescues these defects, preventing embryonic lethality of Rbpms/2-deficient mice, and is sufficient for cardiomyocyte-specific splicing in other cell types.