2025-07-24 京都大学iPS細胞研究所
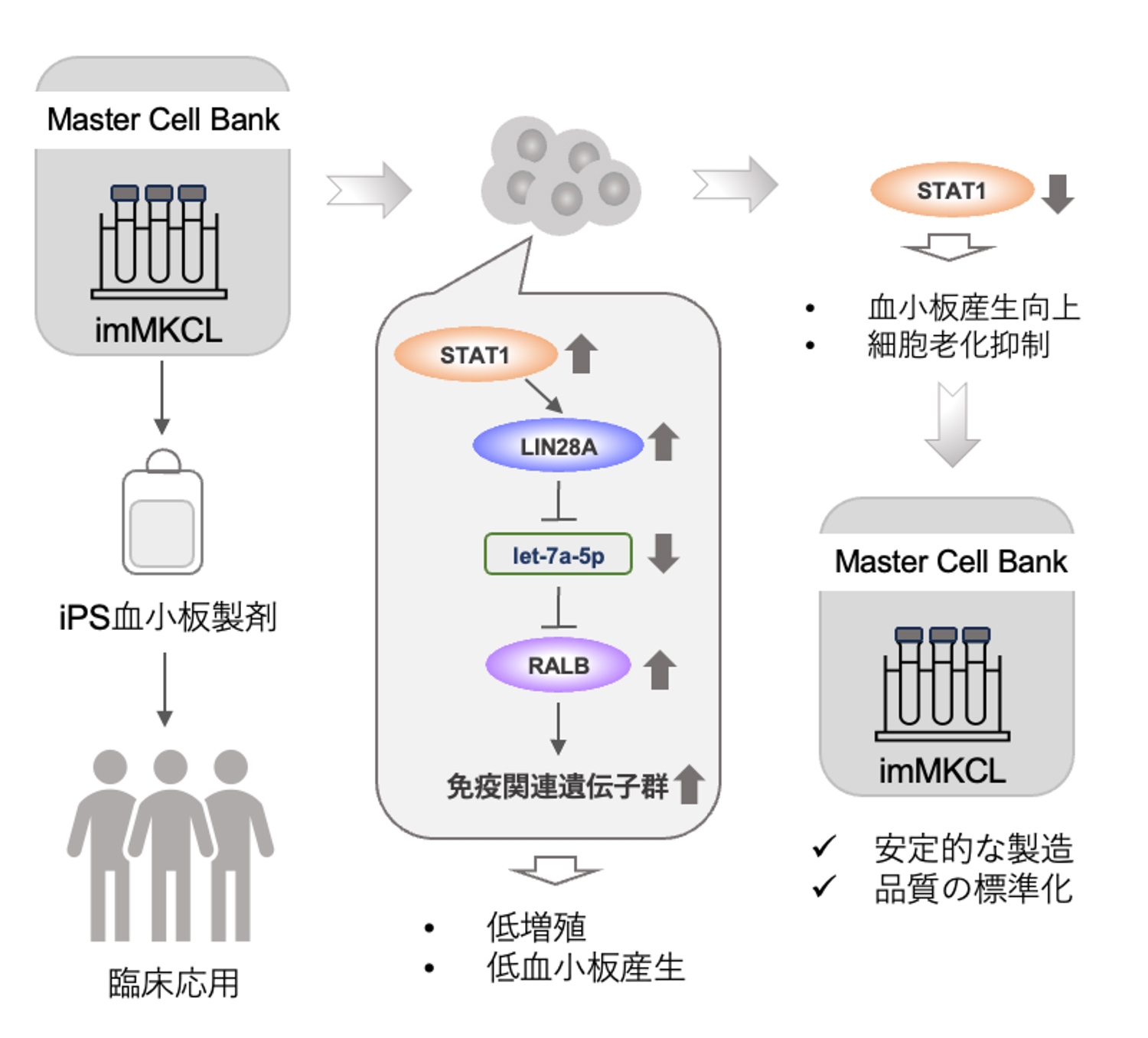 Fig1. 論文の概要図
Fig1. 論文の概要図
<関連情報>
- https://www.cira.kyoto-u.ac.jp/j/pressrelease/news/250724-140000.html
- https://ashpublications.org/bloodadvances/article/doi/10.1182/bloodadvances.2024015557/537795/STAT1-mediated-epigenetic-regulation-of-LIN28A
STAT1を介したエピジェネティックなLIN28A調節が、let-7-RALB軸を介してiPSC由来の血小板産生を制御する STAT1-mediated epigenetic regulation of LIN28A controls iPSC-derived platelet production through the let-7-RALB axis
Kazuya Hashimoto,Si Jing ,Kosuke Fujio,Akihiro Kayama,Naoshi Sugimoto,Naoya Takayama,Moritoki Egi,Koji Eto
Blood Advances Published:January 17, 2025
DOI:https://doi.org/10.1182/bloodadvances.2024015557
Key Points
- LIN28A regulates platelet production through the let-7 microRNA-RALB axis in human iPSC-derived megakaryocyte cell lines.
- STAT1 modulates LIN28A expression via DNA methylation, and its inhibition boosts platelet production while suppressing cellular senescence.
Ex vivo platelet production from induced pluripotent stem cells (iPSCs) represents a potential solution to address the limitations of donor-dependent platelet transfusion therapy. While our established immortalized megakaryocyte progenitor cell lines (imMKCLs) from iPSCs enable the large-scale production of functional iPSC-derived platelet products (iPSC-PLTs), cellular heterogeneity and senescence remain significant challenges for robust industrial-scale manufacturing. We recently identified RAS-like proto-oncogene B (RALB) as a key regulator of immune properties and platelet (PLT) productivity of imMKCLs, acting downstream of the let-7a-5p microRNA. The present study aims to identify the upstream regulators of let-7a-5p in this context. Herein, we demonstrate that the expression of Lin-28 homolog A (LIN28A), which negatively regulates let-7a-5p, is controlled in imMKCLs through DNA methylation-dependent mechanisms. Analysis of the LIN28A locus revealed distinct methylation patterns between let-7high and let-7low populations within an intronic CpG island. Overexpression of LIN28A upregulated immune-related signaling and diminished PLT production from imMKCLs. We further examined for transcriptional regulators by motif enrichment analysis and small interfering RNA (siRNA)-mediated knockdown, identifying signal transducer and activator of transcription 1 (STAT1) as an upstream regulator of LIN28A. Knockdown of STAT1 led to the suppression of immune-related gene expression resulting in increased PLT production. Inhibition of STAT1 phosphorylation with fludarabine and flavopiridol enhanced PLT generation, uncovering a novel role in platelet generation beyond their established functions in cell cycle arrest and apoptosis. In conclusion, our findings unveil the modulating roles of immune and senescence signaling in imMKCLs to optimize cell and culture conditions for large-scale PLT manufacturing.