2025-08-04 京都大学

本研究の概要(Images were provided by Servier Medical Art, NIAID NIH BioArtSource, and TogoTV (© 2016 DBCLS TogoTV), all licensed under CC BY 4.0.)
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-08-04
- https://www.kyoto-u.ac.jp/sites/default/files/2025-08/web_2508_Horie-8972002eb06fa10897e94f5956e0dde2.pdf
- https://www.embopress.org/doi/full/10.1038/s44321-025-00273-9
マイクロRNA-33の阻害は、骨格筋再生を促進することで筋ジストロフィーを改善する MicroRNA-33 inhibition ameliorates muscular dystrophy by enhancing skeletal muscle regeneration
Naoya Sowa, Takahiro Horie, Yuya Ide, Osamu Baba, Kengo Kora, Takeshi Yoshida, Yujiro Nakamura, Shigenobu Matsumura, Kazuki Matsushita, Miyako Imanaka, Fuquan Zou, Eitaro Kume, Hidenori Kojima, Qiuxian Qian, Kayo Kimura, Ryotaro Otsuka, Noriko Hara, Tomohiro Yamasaki, Chiharu Otani, Yuta Tsujisaka, Tomohide Takaya, Chika Nishimura, Dai Watanabe, Koji Hasegawa, Jun Kotera, +7 , and Koh Ono
EMBO Molecular Medicine Published:22 July 2025
DOI:https://doi.org/10.1038/s44321-025-00273-9
Abstract
Muscular dystrophy is a group of diseases characterized by progressive weakness and degeneration of skeletal muscles, for which there is currently no cure. Here, we show that microRNA (miR)-33a/b play a crucial role in muscle regeneration. miR-33a was upregulated during myoblast differentiation and in skeletal muscles of mdx mice, a genetic model of Duchenne muscular dystrophy (DMD). miR-33a deficiency enhanced muscle regeneration response to cardiotoxin injury and attenuated muscle degeneration and fibrosis in mdx mice. Conversely, a humanized mouse model expressing miR-33a and miR-33b showed exacerbated muscle degeneration and fibrosis. Mechanistically, miR-33a/b inhibited satellite cell proliferation, leading to reduced muscle regeneration and increased fibrosis by targeting Cdk6, Fst, and Abca1. Local and systemic administration of anti-miRNA oligonucleotides targeting miR-33a/b ameliorated the dystrophic phenotype in mdx mice. Furthermore, miR-33b inhibition upregulated these target genes in myotubes differentiated from human induced pluripotent stem cells derived from a patient with DMD. These findings indicate that miR-33a/b are involved in muscle regeneration and their inhibition may represent a potential therapeutic strategy for muscular dystrophy.
Synopsis
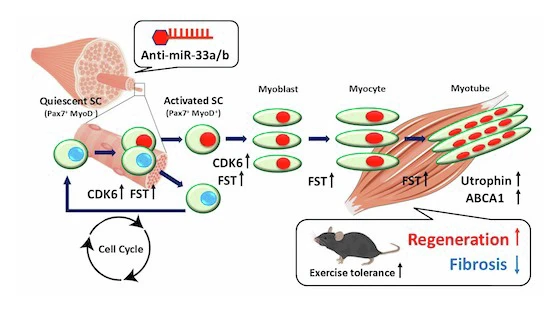
In this study miR-33a/b were identified as negative regulators of skeletal muscle regeneration by targeting Cdk6, Fst, and Abca1. Inhibition of miR-33a/b can be a promising strategy for the treatment of muscular dystrophy including DMD.
- miR-33a knockout accelerated muscle regeneration and ameliorated the dystrophic phenotype in mdx mice.
- miR-33b knock-in exacerbated muscle pathology in mdx mice.
- Pax7+ satellite cell numbers increased in miR-33a-KO and decreased in miR-33b-KI mice.
- Cdk6, Fst, and Abca1 were confirmed as direct targets of miR-33a/b involved in muscle regeneration.
- Antisense inhibition of miR-33a/b improved dystrophic phenotype in mdx mice and upregulated target genes in myotube from DMD patient-derived iPSCs.
The paper explained
Problem
Muscular dystrophy is a class of genetic diseases characterized by progressive skeletal muscle weakness and degeneration. Among them, Duchenne muscular dystrophy (DMD) is one of the most severe and prevalent forms, caused by mutations in the dystrophin gene. Although recent therapeutic advances such as exon skipping have led to partial restoration of dystrophin in specific subsets of DMD patients and supportive care can mildly slow disease progression, no curative treatments are currently available, and overall therapeutic efficacy remains limited.
Results
miR-33a/b were found to play substantial roles in skeletal muscle regeneration. miR-33a-knockout (KO) mice showed accelerated muscle regeneration in response to CTX injury and ameliorated the dystrophic phenotype in mdx mice (widely used genetic DMD models). In contrast, as a gain-of-function, miR-33b knock-in (KI) mice exacerbated the dystrophic phenotype in mdx mice. The number of Pax7+ satellite cells increased in miR-33a-KO mice and decreased in miR-33b-KI mice by targeting Cdk6, Fst, and partially, Abca1. Therapeutic inhibition of miR-33a/b using ASO ameliorated the phenotype of mdx mice. Furthermore, miR-33b inhibition upregulated miR-33 target genes in myotubes from iPS cells derived from a patient with DMD.
Impact
miR-33a/b act as negative regulators of muscle regeneration by suppressing satellite cell expansion. Therapeutical inhibition of miR-33a/b may be a novel therapeutic approach for patients with muscular dystrophy, including DMD.


