2025-08-07 東北大学
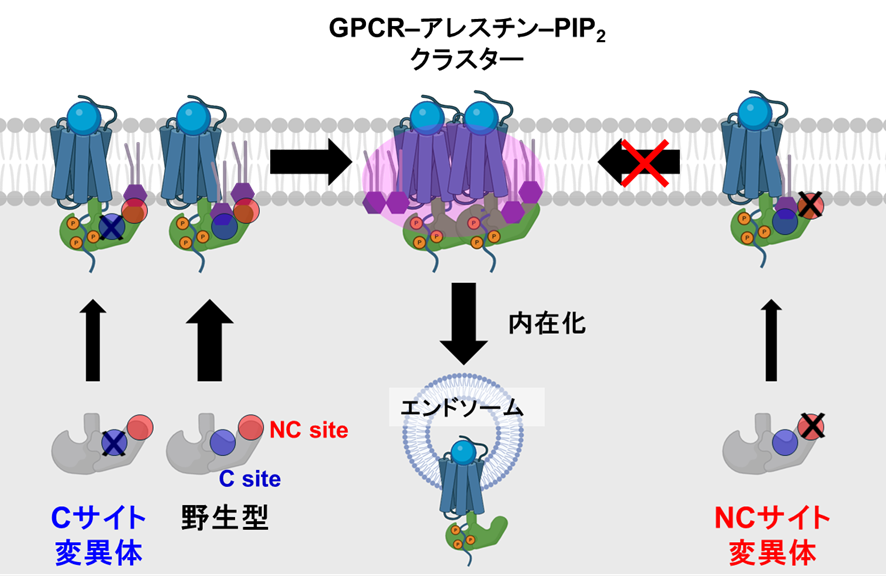 図.アレスチンのNCサイトを介したGPCR内在化の制御機構
図.アレスチンのNCサイトを介したGPCR内在化の制御機構
<関連情報>
- https://www.tohoku.ac.jp/japanese/2025/08/press20250807-03-arrestin.html
- https://www.tohoku.ac.jp/japanese/newimg/pressimg/tohokuuniv-press20250807_03web_arrestin.pdf
- https://www.nature.com/articles/s41589-025-01967-4
β-アレストインによるPtdIns(4,5)P2結合を介した活性GPCRの膜ドメイン区画化 Membrane-domain compartmentalization of active GPCRs by β-arrestins through PtdIns(4,5)P2 binding
Ritsuki Kuramoto,Tatsuya Ikuta,Carlo Marion C. Carino,Kouki Kawakami,Miisha Kushiro,Chihiro Watanabe,Yasunori Uchida,Mitsuhiro Abe,Yasushi Sako,Tomohiko Taguchi,Masataka Yanagawa & Asuka Inoue
Nature Chemical Biology Published:06 August 2025
DOI:https://doi.org/10.1038/s41589-025-01967-4
Abstract
Upon ligand-induced activation, G-protein-coupled receptors (GPCRs) recruit β-arrestins (βarrs) to the plasma membrane, where phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) stabilizes the GPCR–βarr complex. Although PtdIns(4,5)P2 is reported to form nanoscale clusters, the spatiotemporal dynamics of how the GPCR–βarr–PtdIns(4,5)P2 complex assembles and organizes in living cells remain unexplored. Here we demonstrate that multiple PtdIns(4,5)P2-binding sites on βarrs cooperatively promote GPCR–βarr assembly in membrane domains. Using molecular dynamics simulations, we identify a noncanonical (NC) PtdIns(4,5)P2-binding site, distinct from the known canonical site. Biochemical assays confirm that both sites are essential for βarr binding to PtdIns(4,5)P2-containing liposomes, while NanoBiT assays reveal synergistic contributions of both sites for βarr recruitment in living cells. Notably, single-molecule imaging demonstrates that the NC site is required for the rapid accumulation of the GPCR–βarr–PtdIns(4,5)P2 complex into immobile membrane domains upon ligand stimulation. Collectively, our findings highlight how multivalent βarr–PtdIns(4,5)P2 interactions drive GPCR–βarr compartmentalization, adding complexity to GPCR signaling dynamics.