2025-08-28 トロント大学(U of T)
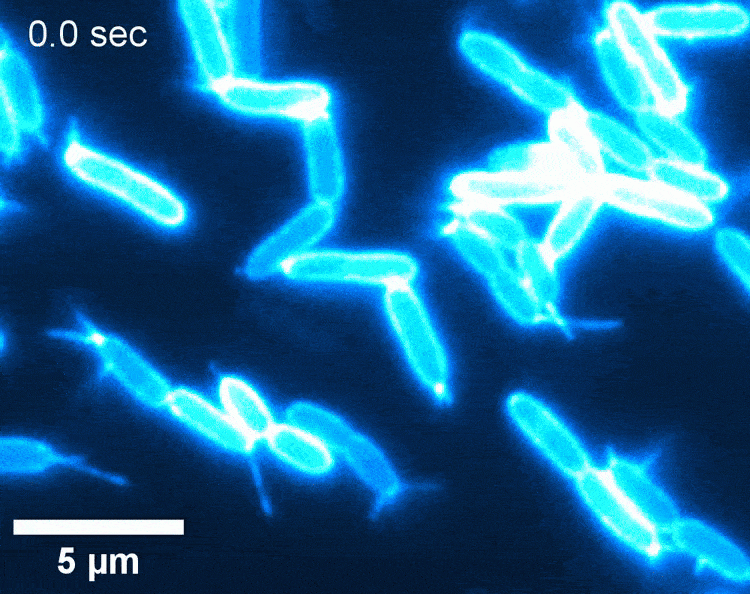
Screenshot from a video showing the movement of fibres called pili – which are located on the bacterial surface and function as a docking station for phages – on typical Pseudomonas bacteria (oval shapes) (video by Matthias Koch)
<関連情報>
- https://www.utoronto.ca/news/u-t-researchers-reveal-how-bacterial-viruses-protect-their-offspring-maximize-spread
- https://www.nature.com/articles/s41586-025-09260-z
プロファージは細胞表面受容体を遮断し、ウイルス子孫を保護する Prophages block cell surface receptors to preserve their viral progeny
Véronique L. Taylor,Pramalkumar H. Patel,Megha Shah,Ahmed Yusuf,Cayla M. Burk,Kristina M. Sztanko,Zemer Gitai,Alan R. Davidson,Matthias D. Koch & Karen L. Maxwell
Nature Published:16 July 2025
DOI:https://doi.org/10.1038/s41586-025-09260-z
Abstract
In microbial communities, viruses compete for host cells and have evolved diverse mechanisms to inhibit competitors. One strategy is superinfection exclusion, whereby an established viral infection prevents a secondary infection of the same cell1. This phenomenon has been shown to have an important role in the spread of eukaryotic viruses. Here we determine that superinfection exclusion proteins in bacterial viruses (bacteriophages, hereafter phages) perform a similar role, promoting viral spread through the bacterial community. We characterize a phage protein that alters the dynamics of a common phage receptor, the type IV pilus. This protein, known as Zip, does not abrogate pilus activity, but fine-tunes it, providing a strong phage defence without a fitness cost. Notably, Zip also prevents internalization and destruction of newly released phage progeny, a phenomenon that we call the anti-Kronos effect after the Greek god who consumed his offspring. Zip activity promotes the accumulation of free phages in bacterial lysogen communities, thereby enhancing viral spread. We further demonstrate that the anti-Kronos effect is conserved across diverse prophage-encoded superinfection exclusion systems. Our results identify the mechanistic basis of a superinfection exclusion system that safeguards phage progeny and provide insights into the conservation of viral defence mechanisms among bacterial and eukaryotic systems.


