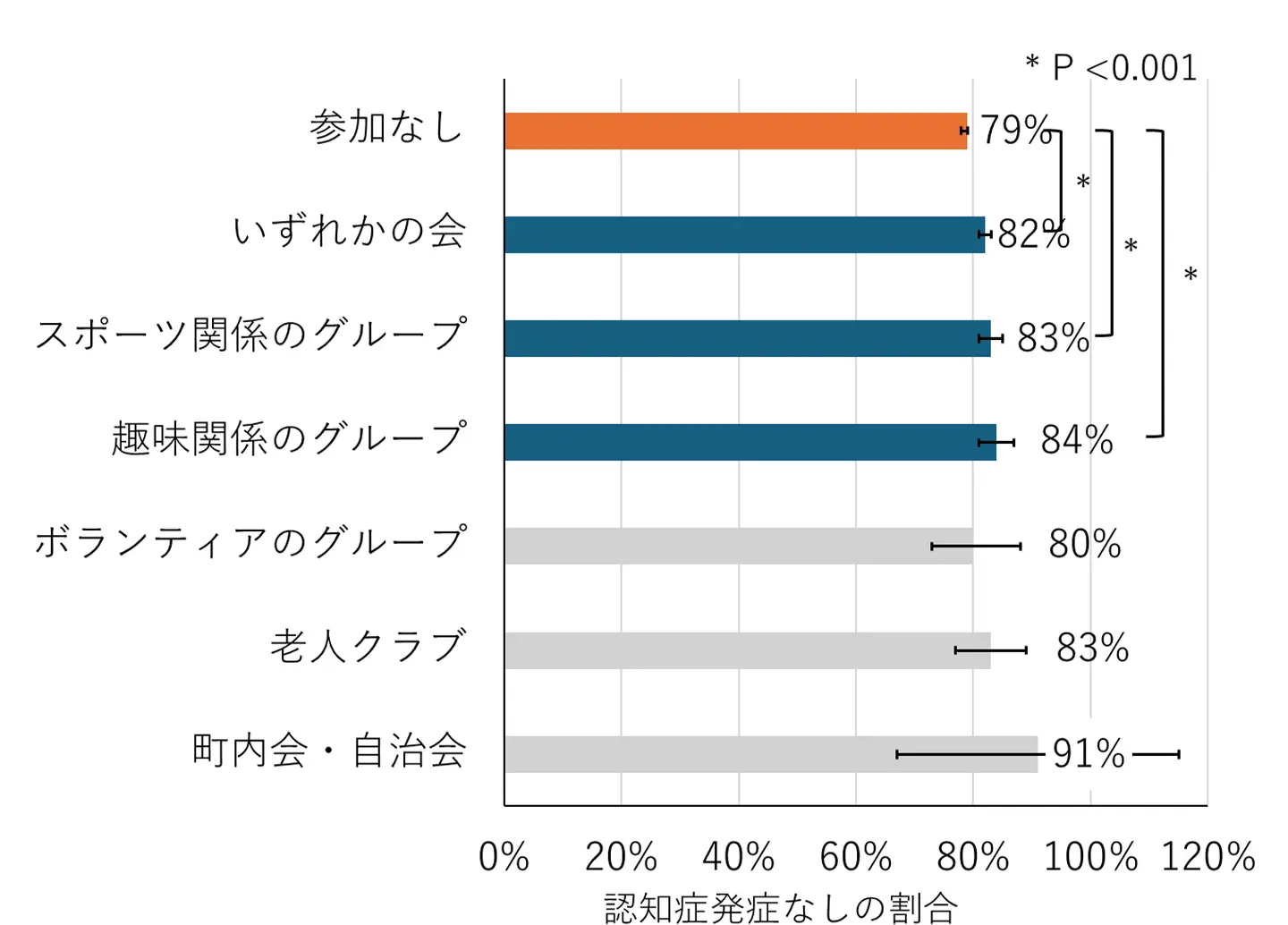
2025-10-22 東京科学大学
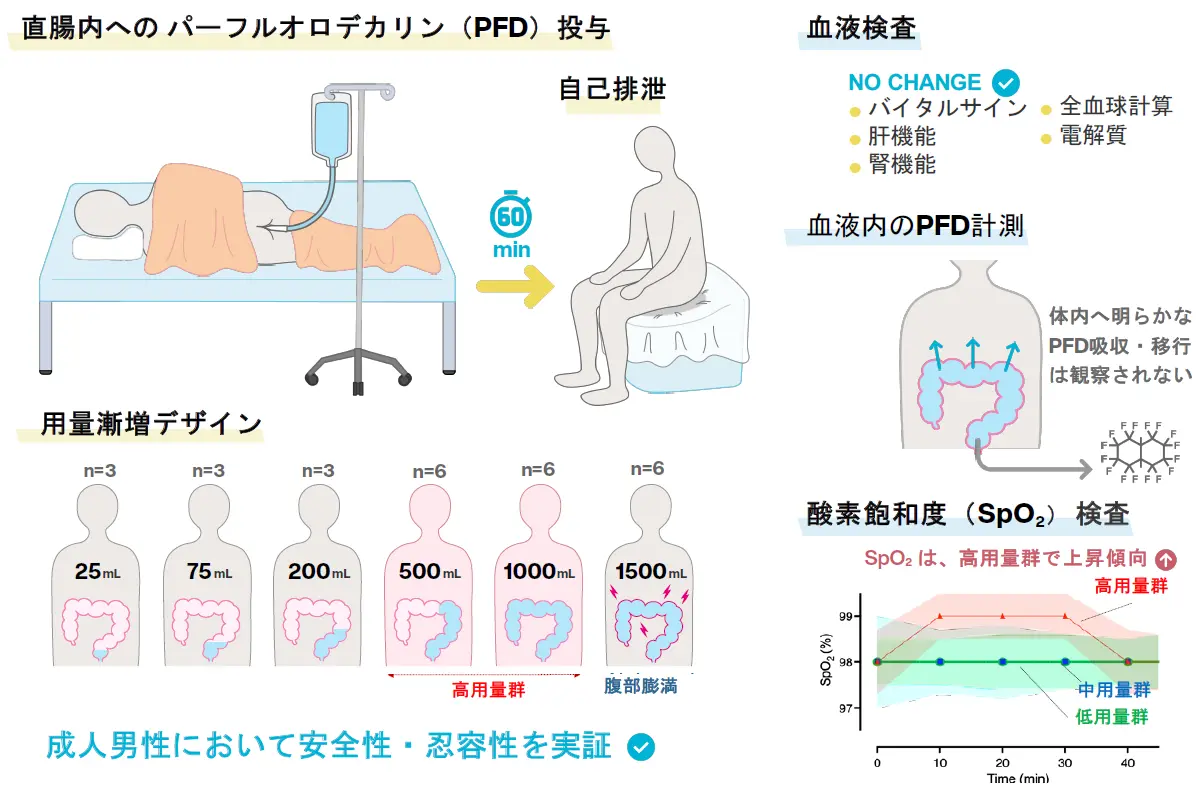
図1. 本臨床試験のデザインと成果概要. 健康な成人男性を対象に、3+3デザインと呼ばれる手法で、安全性を確認しながら段階的にPFDの投与量を増やしていく「用量漸増試験」を実施した。本試験により、最大1,500 mLまでの投与における安全性と忍容性が評価された。
<関連情報>
ヒト初回試験における経腸換気のための直腸内ペルフルオロデカリンの安全性と忍容性 Safety and tolerability of intrarectal perfluorodecalin for enteral ventilation in a first-in-human trial
Tasuku Fujii ∙ Yasuyuki Kurihara ∙ Yoshihiko Tagawa ∙ … ∙ Chihiro Yokota ∙ Hiroyuki Mizuo ∙ Takanori Takebe
Med Published:October 20, 2025
DOI:https://doi.org/10.1016/j.medj.2025.100887
Context and significance
Patients with severe respiratory failure often need mechanical ventilation to survive, but these therapies can cause further lung injury. Scientists are exploring a new method called “enteral ventilation” to deliver oxygen through the intestine, which could give the lungs a chance to rest and heal. This study evaluated the safety of this method in humans for the first time, using a special liquid called perfluorodecalin with exceptional oxygen-carrying ability. In a trial with 27 healthy male volunteers, the authors found that administering this liquid rectally was safe and well tolerated. This important safety milestone paves the way for future studies to see if this technique can help patients with respiratory failure.
Highlights
- First-in-human, dose-escalation trial for intrarectal perfluorodecalin (PFD)
- Favorable tolerability profile up to 1,000 mL PFD, with all adverse events being mild
- No detectable systemic absorption of PFD (<1.0 μg/mL)
- Dose-dependent oxygen transfer predicted by pig pharmacokinetic model
Summary
Background
Enteral ventilation is an emerging approach that provides partial systemic oxygenation independent of pulmonary gas exchange, enabling lung rest. Perfluorodecalin, a clinically approved liquid with high oxygen solubility, is a promising vehicle for enteral oxygen delivery. The primary endpoint of this first-in-human trial was to assess the safety and tolerability of intrarectal perfluorodecalin administration.
Methods
This was a phase 1, single-site, open-label, non-controlled, dose-escalation trial in 27 healthy adult males aged 20–45 years. Participants received a single intrarectal dose of non-oxygenated perfluorodecalin (escalating from 25 to 1,500 mL) retained for 60 min. Safety and tolerability were assessed through monitoring of adverse events, vital signs, clinical laboratory tests, and systemic perfluorodecalin exposure. A pharmacokinetic model using large-animal data was employed to predict potential oxygen transfer.
Findings
No serious adverse events or dose-limiting toxicities occurred. Mild gastrointestinal symptoms, such as abdominal bloating and pain, were transient, dose dependent, and resolved without intervention. All clinical laboratory parameters, including liver and renal function markers, remained within normal limits. Perfluorodecalin concentrations were undetectable in blood (<1.0 μg/mL). The pharmacokinetic model predicted a dose-dependent oxygenation effect, consistent with a modest increase in peripheral oxygen saturation observed in the higher-dose group.
Conclusions
This first-in-human study demonstrates that intrarectal administration of non-oxygenated perfluorodecalin is safe, feasible, and well tolerated. These findings establish a critical safety foundation and support the continued development of enteral ventilation with fully oxygenated perfluorodecalin as an adjunctive strategy to support respiratory failure patients.
Funding
This work was funded by EVA Therapeutics, Inc.