2025-10-30 マウントサイナイ医療システム(MSHS)
Web要約 の発言:
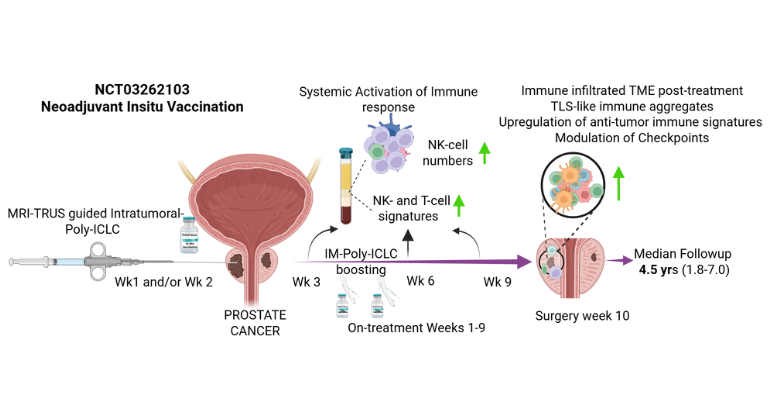
A novel pre-surgery therapy triggered immune responses in prostate tumors and produced encouraging early tumor changes, offering a potential new path for immunotherapy. Image credit: Nair et al., Med
<関連情報>
- https://www.mountsinai.org/about/newsroom/2025/early-clinical-trial-tests-immune-boosting-therapy-before-prostate-cancer-surgery
- https://www.cell.com/med/fulltext/S2666-6340%2825%2900306-X
腫瘍内ウイルス模倣ポリICLCを用いた前立腺癌in situ自己ワクチン接種:低温腫瘍微小環境の調節 Prostate cancer in situ autovaccination with the intratumoral viral mimic poly-ICLC: Modulating the cold tumor microenvironment
Sujit S. Nair ∙ Dimple Chakravarty ∙ Sreekumar Balan ∙ … ∙ Andres M. Salazar ∙ Nina Bhardwaj ∙ Ashutosh K. Tewari
Med Published:October 30, 2025
DOI:https://doi.org/10.1016/j.medj.2025.100879
Context and significance
Many patients with high-risk prostate cancer experience recurrence after surgery, and it remains challenging to treat with immunotherapy because tumors typically show low immune activity. This is the first study in prostate cancer to evaluate the safety of a pre-surgery (neoadjuvant) approach designed to activate the immune system to fight cancer, in which a virus-mimicking drug (poly-ICLC) is injected into the tumor to start an immune response and then into a muscle to boost it. The treatment was safe and showed early signs of immune activation in blood and tumor samples, including favorable changes in gene expression. By demonstrating feasibility and immune engagement, this approach lays groundwork for future studies to prime the immune system against prostate cancer before surgery.
Highlights
- Study addresses a key challenge in prostate cancer: poor response to immunotherapy
- Poly-ICLC was safely delivered intratumorally and intramuscularly before prostatectomy
- Treatment increased immune infiltration, checkpoint levels, and favorable gene shifts
- In situ autovaccination primed antitumor immunity, supporting future combination trials
Summary
Background
Neoadjuvant therapies for high-risk PCa have shown promise but remain confined to clinical trials. Translating neoadjuvant approaches into routine care thus underscores the critical need for innovative early-phase neoadjuvant trials to evaluate safety and efficacy in localized disease, where tumors are more responsive to intervention.
Methods
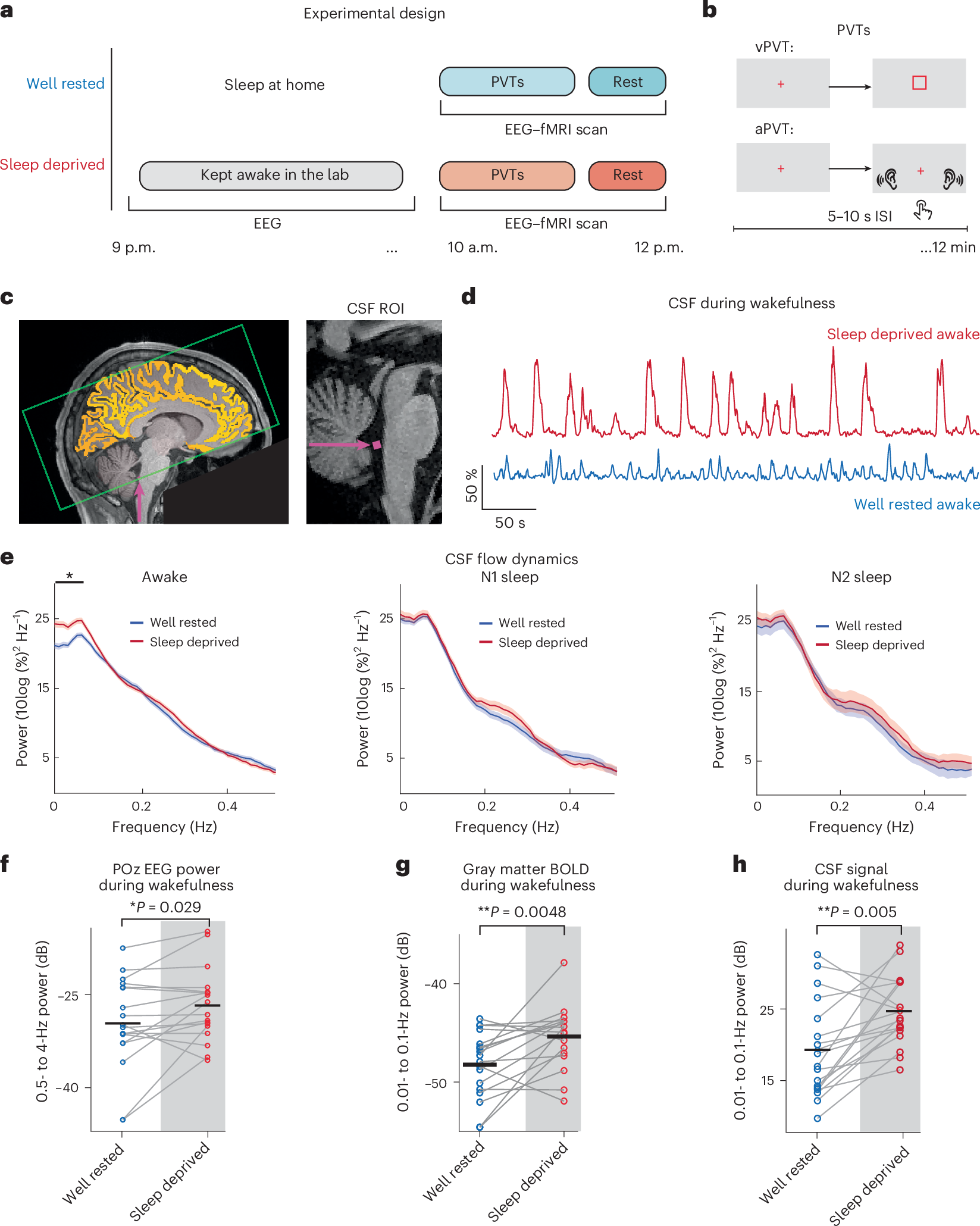
In this open-label phase 1 trial (NCT03262103), 12 patients with clinically localized intermediate- to high-risk PCa scheduled for radical prostatectomy (RP) received sequential intratumoral and intramuscular injections of poly-ICLC (Hiltonol®), with the primary endpoint to define a safe dose and schedule and one of the secondary endpoints to characterize associated adverse events.
Findings
All patients tolerated poly-ICLC without dose-limiting toxicity or treatment withdrawal. Median follow-up was 4.5 years. Seventy percent of the evaluable patients had PSA0 (measured as PSA <0.1 ng/mL) 1 year post-RP. Gleason score at final pathology was downgraded in 66.7% of all patients and 70% of the high-risk subgroup. Tissue transcriptomic analysis revealed decreased metastasis signature post-treatment, with upregulation of immune cell-related and favorable-prognosis genes. Intratumoral and intramuscular poly-ICLC also enhanced immune activation signatures in the blood and increased NK cells in both blood and tissues. Treatment increased post-treatment infiltration of CD4+, CD8+, and PD-1+ T cells; CD56+ NK cells; CD20+ B cells; and tertiary lymphoid structure-like aggregates.
Conclusions
Intratumoral poly-ICLC immunotherapy for PCa is safe and may modulate the tumor microenvironment, enhancing antitumor responses. These findings support larger, controlled trials to assess effects on long-term clinical outcomes.
Funding
This work was funded by the Arthur M. Blank Family Foundation.