2025-11-12 カリフォルニア大学リバーサイド校 (UCR)
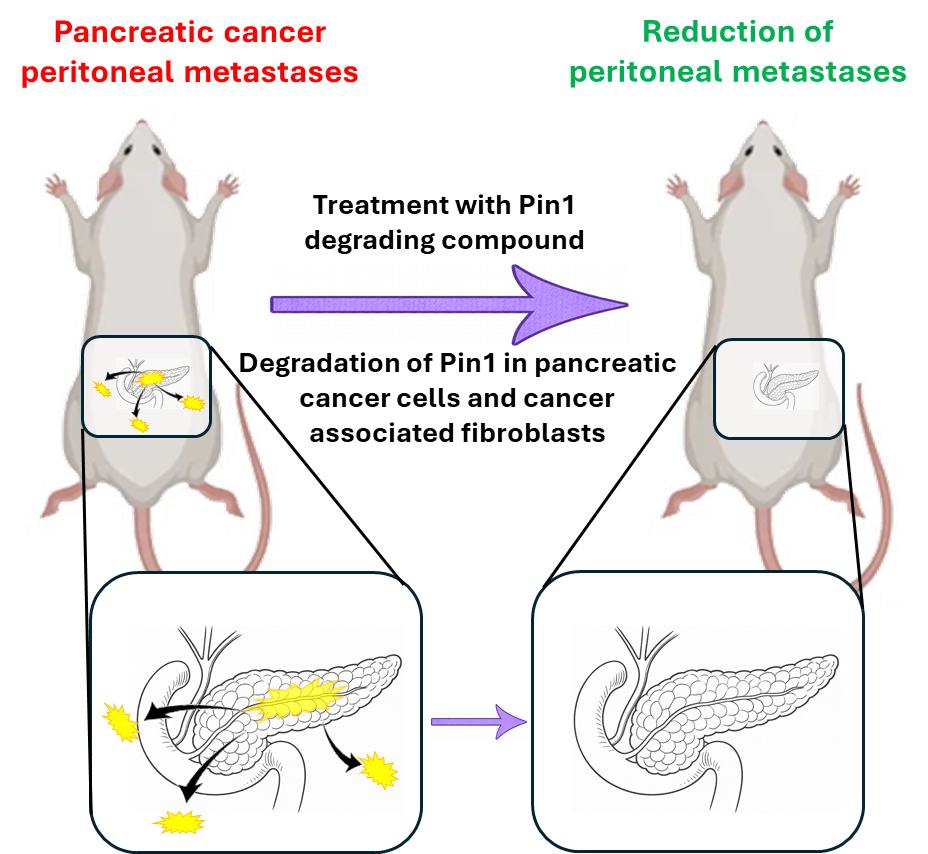
Tumor and peritoneal metastases are shown in yellow. (UCR/Pellecchia lab)
<関連情報>
- https://news.ucr.edu/articles/2025/11/12/scientists-move-closer-better-pancreatic-cancer-treatments
- https://www.cell.com/molecular-therapy-family/oncology/fulltext/S2950-3299(25)00147-X
膵臓癌における強力かつ効果的なPin1分解剤の前臨床評価 Pre-clinical evaluation of a potent and effective Pin1-degrading agent in pancreatic cancer
Giulia Alboreggia ∙ Tiane Li ∙ Anne Marie Prentiss ∙ … ∙ Mingye Feng ∙ Mustafa Raoof ∙ Maurizio Pellecchia
Molecular Therapy Oncology Published:October 31, 2025
DOI:https://doi.org/10.1016/j.omton.2025.201078
Abstract
The peptidyl-prolyl isomerase protein Pin1 has been shown to contribute to cancer onset, development, and progression by regulating the function and stability of oncogenes and tumor suppressors, both in cancer cells and surrounding stromal cells. Hence, it has long been sought as a possible therapeutic target in oncology. However, further validation of Pin1 as a suitable target for novel therapeutic strategies requires the development of potent and effective pharmacological inhibitors, which are currently scarce. Very recently, we reported novel covalent Pin1 inhibitors that, acting as molecular crowbars, destabilize the protein in vitro, causing its degradation in cells. Here, we advanced those agents by improving their plasma stability. We further characterized their mode of action on cancer cells and cancer-associated fibroblasts and studied their efficacy in a syngeneic mouse model of pancreatic cancer peritoneal metastases. Our studies support the hypothesis that these novel Pin1 degraders could be translated into effective therapeutics against peritoneal metastases in pancreatic and other types of gastrointestinal and abdominal cancers that develop peritoneal metastases.