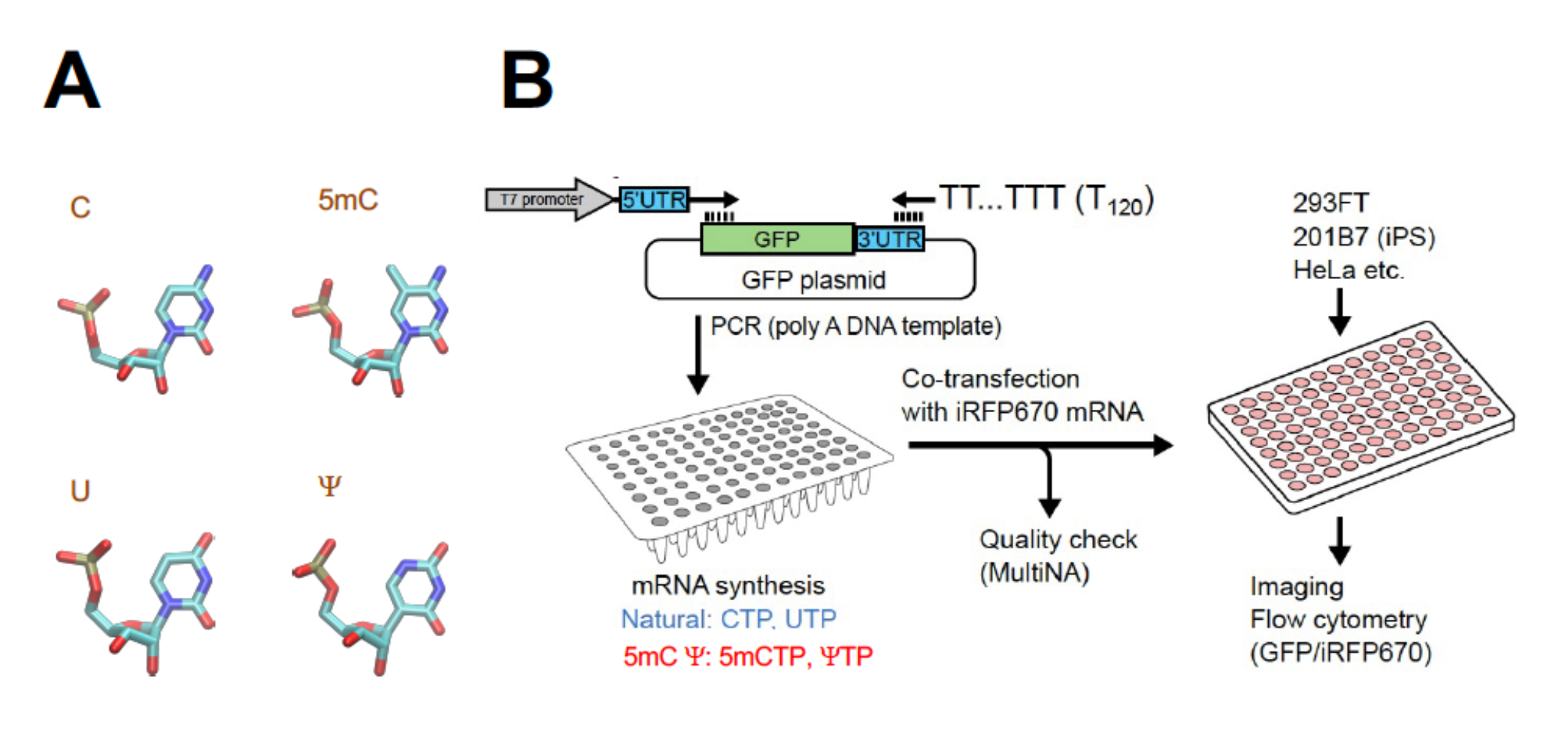
シングルセル技術と機械学習の組み合わせにより、がん細胞と免疫系のコラボレーションを発見。 A combination of single-cell techniques and machine learning uncovers a collaboration between cancer cells and the immune system.
2022-04-11 フィンランド・アールト大学
<関連情報>
- https://www.aalto.fi/en/news/blood-cancer-cells-and-the-immune-system-are-best-frenemies
- https://www.nature.com/articles/s41467-022-29173-z
CD8+ T細胞性大顆粒リンパ球性白血病における白血病および非白血病性免疫レパートリーの単細胞特性化 Single-cell characterization of leukemic and non-leukemic immune repertoires in CD8+ T-cell large granular lymphocytic leukemia
Jani Huuhtanen,Dipabarna Bhattacharya,Tapio Lönnberg,Matti Kankainen,Cassandra Kerr,Jason Theodoropoulos,Hanna Rajala,Carmelo Gurnari,Tiina Kasanen,Till Braun,Antonella Teramo,Renato Zambello,Marco Herling,Fumihiro Ishida,Toru Kawakami,Marko Salmi,Thomas Loughran,Jaroslaw P. Maciejewski,Harri Lähdesmäki,Tiina Kelkka &Satu Mustjoki
Nature Communications Published: 11 April 2022
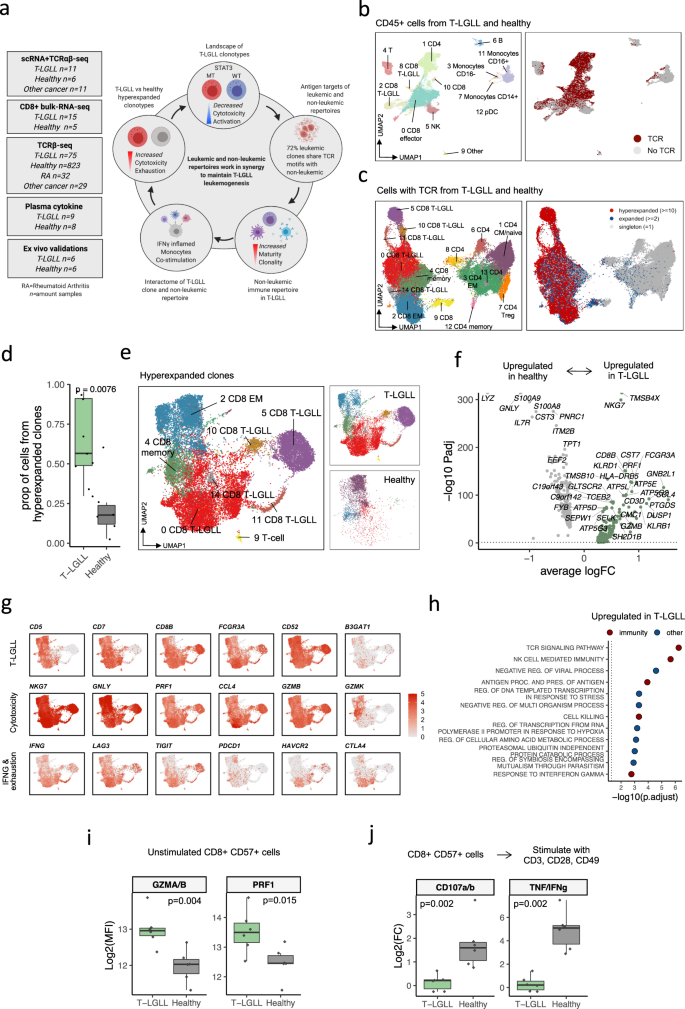
Fig. 1: T-LGLL細胞は、健康な過剰増殖型クロノタイプと比較して、高い細胞傷害性と消耗性を示している。
Abstract
T cell large granular lymphocytic leukemia (T-LGLL) is a rare lymphoproliferative disorder of mature, clonally expanded T cells, where somatic-activating STAT3 mutations are common. Although T-LGLL has been described as a chronic T cell response to an antigen, the function of the non-leukemic immune system in this response is largely uncharacterized. Here, by utilizing single-cell RNA and T cell receptor profiling (scRNA+TCRαβ-seq), we show that irrespective of STAT3 mutation status, T-LGLL clonotypes are more cytotoxic and exhausted than healthy reactive clonotypes. In addition, T-LGLL clonotypes show more active cell communication than reactive clones with non-leukemic immune cells via costimulatory cell–cell interactions, monocyte-secreted proinflammatory cytokines, and T-LGLL-clone-secreted IFNγ. Besides the leukemic repertoire, the non-leukemic T cell repertoire in T-LGLL is also more mature, cytotoxic, and clonally restricted than in other cancers and autoimmune disorders. Finally, 72% of the leukemic T-LGLL clonotypes share T cell receptor similarities with their non-leukemic repertoire, linking the leukemic and non-leukemic repertoires together via possible common target antigens. Our results provide a rationale to prioritize therapies that target the entire immune repertoire and not only the T-LGLL clonotype.