ヒト幹細胞由来の臓器構成ブロックを用いてバイオプリントした心臓組織の機能層に、収縮力をプログラムできる新たな組織工学の可能性を発見 New tissue engineering capabilities enable researchers to program contractility in functional layers of heart tissue bioprinted with human stem cell-derived organ building blocks
2022-06-10 ハーバード大学
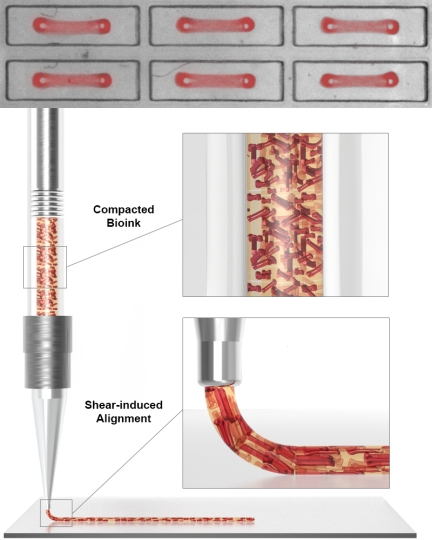
To create layers of aligned contractile human heart muscle tissue, the team first developed a platform with which they could simultaneously generate 1050 individual contractile Organ Building Blocks (OBBs) between two micropillars, using human induced Pluripotent Stem Cells (hiPSCs), human fibroblast cells and essential extracellular matrix molecules (ECMs). The OBBs were then lifted off the micropillars and used as a feedstock to fabricate a dense bioink (lower panel). In a second alignment step the mechanical shear forces generated at the print head during the printing process act on the OBBs while they are being extruded to give them directionality in printed cardiac tissue sheets. Credit: Wyss Institute at Harvard University
<関連情報>
- https://www.seas.harvard.edu/news/2022/06/getting-heart-engineering-heart
- https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202200217
異方性器官ブロックのバイオプリントによる人工心筋組織における細胞配列のプログラミング Programming Cellular Alignment in Engineered Cardiac Tissue via Bioprinting Anisotropic Organ Building Blocks
John H. Ahrens,Sebastien G. M. Uzel,Mark Skylar-Scott,Mariana M. Mata,Aric Lu,Katharina T. Kroll,Jennifer A. Lewis
Advanced Materials Published: 21 April 2022
DOI:https://doi.org/10.1002/adma.202200217
Abstract
The ability to replicate the 3D myocardial architecture found in human hearts is a grand challenge. Here, the fabrication of aligned cardiac tissues via bioprinting anisotropic organ building blocks (aOBBs) composed of human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) is reported. A bioink composed of contractile cardiac aOBBs is first generated and aligned cardiac tissue sheets with linear, spiral, and chevron features are printed. Next, aligned cardiac macrofilaments are printed, whose contractile force and conduction velocity increase over time and exceed the performance of spheroid-based cardiac tissues. Finally, the ability to spatially control the magnitude and direction of contractile force by printing cardiac sheets with different aOBB alignment is highlighted. This research opens new avenues to generating functional cardiac tissue with high cell density and complex cellular alignment.