数十億年前の酵素がよみがえり、光合成が酸素の増加にどのように適応してきたかが明らかになった Resurrecting billon-year-old enzymes reveals how photosynthesis adapted to the rise of oxygen
2022-10-10 マックス・プランク研究所
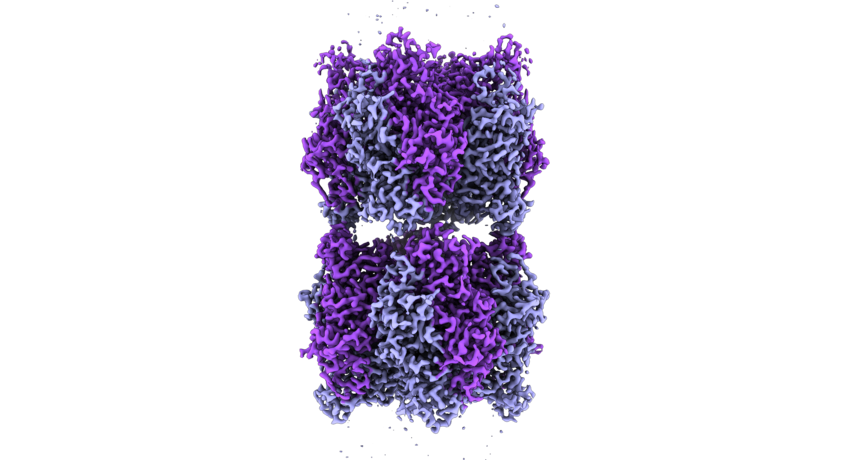
Cryo-electron microscope image of two Rubisco complexes interacting with each other. If a subunit essential for solubility is missing, individual enzyme complexes can interact with each other in this way and form thread-like structures, so-called fibrils. Under normal conditions, however, Rubisco does not form such fibrils.
© MPI f. Terrestrial Microbiology/ L. Schulz
マックス・プランクの研究者チームは、10億年前の酵素を復元することによって、初期の光合成の重要な適応の一つを解読した。この成果は、現代の光合成の進化に関する洞察をもたらすだけでなく、光合成を改善するための新たなヒントを与えてくれるものである。
研究チームは、計算と合成のアプローチを組み合わせて、10億年前の酵素を実験室で復活させ、研究することに成功した。研究者らはこのプロセスを「分子古生物学」と呼んでいるが、その結果、活性中心が直接変異したのではなく、全く新しいコンポーネントが光合成を準備し、酸素レベルの上昇に適応していることを発見したのである。
<関連情報>
- https://www.mpg.de/19348003/1010-terr-back-to-the-future-of-photosynthesis-153410-x?c=2249
- https://www.science.org/doi/10.1126/science.abq1416
形態Iルビスコの黎明期における複雑性と特異性の増大の進化 Evolution of increased complexity and specificity at the dawn of form I Rubiscos
Luca Schulz ,Zhijun Guo,Jan Zarzycki ,Wieland Steinchen ,Jan M. Schuller ,Thomas Heimerl,Simone Prinz ,Oliver Mueller-Cajar ,Tobias J. Erb,Georg K. A. Hochberg
Science Published:13 Oct 2022
DOI: 10.1126/science.abq1416
RELATED PERSPECTIVE
Reconstructing CO2 fixation from the past
BY ROBERT E. SHARWOOD
Origins of Rubisco’s small subunit sidekick
Maintaining the correct activity, specificity, and stability is a challenge faced by metabolic enzymes that is often solved by forming multimeric complexes. The large, catalytic subunit of form I Rubisco, the carbon-fixing enzyme found in aerobic photosynthetic organisms such as plants and cyanobacteria and in some nonphotosynthetic bacteria, makes such a complex with an essential small subunit. Using ancestral sequence reconstruction, Schulz et al. investigated how recruiting this small subunit contributed to Rubisco evolution and, in particular, its specificity for carbon dioxide (see the Perspective by Sharwood). Enzyme activity assays and macromolecular structures revealed that the small subunit quickly became essential and opened the door to broader functional changes within the large subunit, including increased activity and specificity for carbon dioxide. —MAF
Abstract
The evolution of ribulose-1,5-bisphosphate carboxylase/oxygenases (Rubiscos) that discriminate strongly between their substrate carbon dioxide and the undesired side substrate dioxygen was an important event for photosynthetic organisms adapting to an oxygenated environment. We use ancestral sequence reconstruction to recapitulate this event. We show that Rubisco increased its specificity and carboxylation efficiency through the gain of an accessory subunit before atmospheric oxygen was present. Using structural and biochemical approaches, we retrace how this subunit was gained and became essential. Our work illuminates the emergence of an adaptation to rising ambient oxygen levels, provides a template for investigating the function of interactions that have remained elusive because of their essentiality, and sheds light on the determinants of specificity in Rubisco.