2023-02-13 カリフォルニア大学バークレー校(UCB)

Within about a week, the spores of the Magnaporthe fungus grow on and invade rice leaves, establish themselves inside and produce more spores to spread the infection across the rice field. (Graphic credit: Wilson and Talbot, Nature Reviews Microbiology)
◆いもち病は、真菌であるMagnaporthe oryzaeによって引き起こされ、天候にもよるが、世界の稲作の10%から35%を占める植物を毎年攻撃し、枯死させる。
◆カリフォルニア大学バークレー校の化学および分子細胞生物学のMichael Marletta教授率いる生化学者らは、この菌が酵素を分泌し、イネの葉の丈夫な外皮に穴を開けることを発見した。いったん内部に侵入すると、菌は急速に成長し、必然的に植物を枯らしてしまう。
◆『Proceedings of the National Academy of Sciences』誌に掲載された論文で、マレッタ教授らは、この酵素の構造と、菌が植物に侵入する際にどのように作用するかを説明している。この酵素は稲の葉の表面に分泌されるため、植物の壁を消化する酵素の能力を破壊するには、簡単なスプレーが効果的であると考えられる。研究者たちは現在、酵素を阻害する化学物質をスクリーニングしているところです。
◆この酵素は、多糖類モノオキシゲナーゼ(PMO)と呼ばれる酵素群の1つで、マレッタとカリフォルニア大学バークレー校の同僚たちが、10年余り前に、より広く分布する別の真菌、ノイロスポラから発見したものだ。多糖類とは、デンプンや、セルロース、リグニンなど、植物を丈夫にしている繊維を含む糖のポリマーである。PMO酵素はセルロースを細かく分解し、セルラーゼなどの他の酵素の影響を受けやすくして、植物繊維の分解を早める。
<関連情報>
- https://news.berkeley.edu/2023/02/13/discovery-could-lead-to-new-fungicides-to-protect-rice-crops/
- https://www.pnas.org/doi/10.1073/pnas.2215426120
植物病原体Magnaporthe oryzae由来のユニークな多糖類モノオキシゲナーゼの特性評価 Characterization of a unique polysaccharide monooxygenase from the plant pathogen Magnaporthe oryzae
Alejandra Martinez-D’Alto,Xia Yan,Tyler C. Detomasi,Richard I. Sayler,William C. Thomas,Nicholas J. Talbot,Michael A. Marletta
Proceedings of the National Academy of Sciences Published:February 15, 2023
DOI:https://doi.org/10.1073/pnas.2215426120
Significance
Blast disease is a worldwide concern affecting crops like rice and wheat. During plant penetration, the causative fungus Magnaporthe oryzae secretes a polysaccharide monooxygenase (MoPMO9A). Here, we show that MoPMO9A is active on (1→3, 1→4)-β-glucans present in the cell wall of cereal-type plants, hydroxylating the C4 position of the glycosidic bond and using either oxygen or hydrogen peroxide as a cosubstrate. This PMO has a second domain of unknown function that is highly conserved within a subset of PMOs and is essential for PMO activity. Moreover, MoPMO9A deletion results in reduced pathogenicity in rice. Taken together, this work provides insight into the biochemistry of MoPMO9A and the domain architecture of PMOs, supporting its role in polysaccharide degradation during plant infection.
Abstract
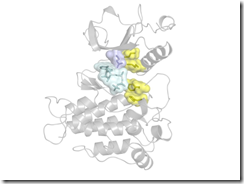
Blast disease in cereal plants is caused by the fungus Magnaporthe oryzae and accounts for a significant loss in food crops. At the outset of infection, expression of a putative polysaccharide monooxygenase (MoPMO9A) is increased. MoPMO9A contains a catalytic domain predicted to act on cellulose and a carbohydrate-binding domain that binds chitin. A sequence similarity network of the MoPMO9A family AA9 showed that 220 of the 223 sequences in the MoPMO9A-containing cluster of sequences have a conserved unannotated region with no assigned function. Expression and purification of the full length and two MoPMO9A truncations, one containing the catalytic domain and the domain of unknown function (DUF) and one with only the catalytic domain, were carried out. In contrast to other AA9 polysaccharide monooxygenases (PMOs), MoPMO9A is not active on cellulose but showed activity on cereal-derived mixed (1→3, 1→4)-β-D-glucans (MBG). Moreover, the DUF is required for activity. MoPMO9A exhibits activity consistent with C4 oxidation of the polysaccharide and can utilize either oxygen or hydrogen peroxide as a cosubstrate. It contains a predicted 3-dimensional fold characteristic of other PMOs. The DUF is predicted to form a coiled-coil with six absolutely conserved cysteines acting as a zipper between the two α-helices. MoPMO9A substrate specificity and domain architecture are different from previously characterized AA9 PMOs. The results, including a gene ontology analysis, support a role for MoPMO9A in MBG degradation during plant infection. Consistent with this analysis, deletion of MoPMO9A results in reduced pathogenicity.