2023-03-09 ペンシルベニア州立大学(PennState)
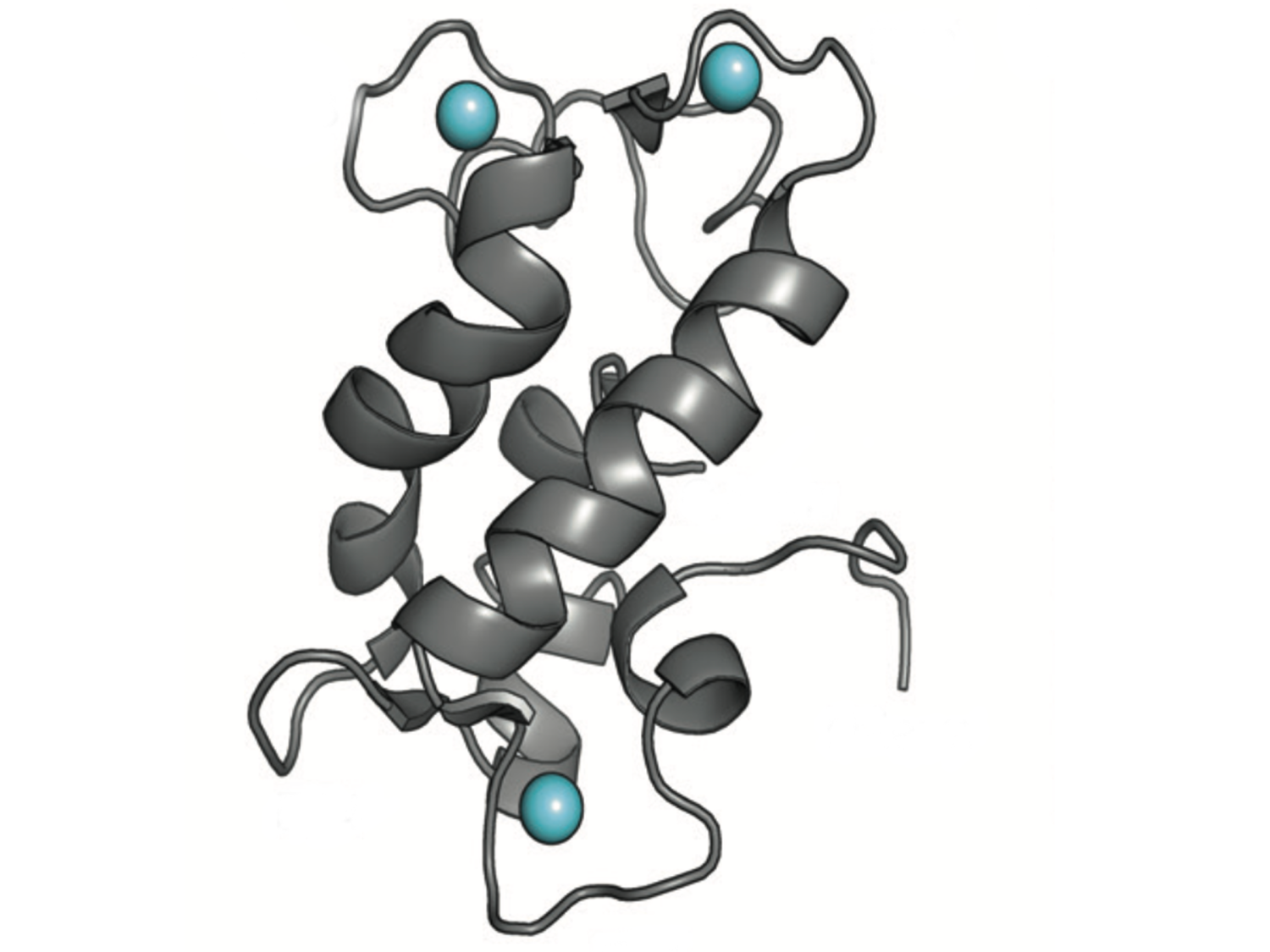 Nuclear magnetic resonance shows the structure of a natural protein called lanmodulin, which binds rare earth elements with high selectivity and was discovered 5 years ago by Penn State researchers. Researchers recently genetically reprogramed the protein to favor manganese over other common transition metals like iron and copper. Credit: Courtesy Cotruvo Lab / Penn State. Creative Commons
Nuclear magnetic resonance shows the structure of a natural protein called lanmodulin, which binds rare earth elements with high selectivity and was discovered 5 years ago by Penn State researchers. Researchers recently genetically reprogramed the protein to favor manganese over other common transition metals like iron and copper. Credit: Courtesy Cotruvo Lab / Penn State. Creative Commons
研究者たちは、選択性が高く、5年前に発見されたレアアースエレメントに結合する天然タンパク質であるランモジュリンからセンサーを作り上げました。センサーは、光合成、宿主-病原体相互作用、神経生物学などの生物技術に広範な応用があります。また、リチウムイオンバッテリーのリサイクルなどのプロセスにも適用することができます。
研究者たちは、最近この発見をProceedings of the National Academy of Sciencesで公表しました。
<関連情報>
- https://www.psu.edu/news/research/story/new-biosensor-reveals-activity-elusive-metal-thats-essential-life/
- https://www.pnas.org/doi/10.1073/pnas.2212723119
ランモジュリンから設計されたマンガン(II)の遺伝子組換え蛍光センサー A genetically encoded fluorescent sensor for manganese(II), engineered from lanmodulin
Jennifer Park,Michael B. Cleary,Danyang Li,Joseph A. Mattocks,Jiansong Xu,Huan Wang,Somshuvra Mukhopadhyay,Eric M. Gale,Joseph A. Cotruvo Jr
Proceedings of the National Academy of Sciences Published:December 12, 2022
DOI:https://doi.org/10.1073/pnas.2212723119
Significance
Virtually no chemical biology tools exist for real-time imaging of manganese(II) in cells. Such tools could help to understand how manganese functions in oxidative defense, photosynthesis, and other important biological processes, both when protein-bound as an enzyme cofactor and unbound in the labile manganese pool. Here we show that a recently discovered native lanthanide-binding protein can be re-engineered to respond to manganese with strong selectivity over the most important interfering metals in cells (magnesium, iron, and calcium). This genetically encoded fluorescent sensor reports manganese fluxes in bacterial cells in real time, laying the foundation for a new approach to studying manganese physiology. More broadly, it suggests general strategies for re-engineering non-native metal selectivity into proteins for wide-ranging applications.
Abstract
The design of selective metal-binding sites is a challenge in both small-molecule and macromolecular chemistry. Selective recognition of manganese (II)—the first-row transition metal ion that tends to bind with the lowest affinity to ligands, as described by the Irving-Williams series—is particularly difficult. As a result, there is a dearth of chemical biology tools with which to study manganese physiology in live cells, which would advance understanding of photosynthesis, host-pathogen interactions, and neurobiology. Here we report the rational re-engineering of the lanthanide-binding protein, lanmodulin, into genetically encoded fluorescent sensors for MnII, MnLaMP1 and MnLaMP2. These sensors with effective Kd(MnII) of 29 and 7 µM, respectively, defy the Irving-Williams series to selectively detect MnII in vitro and in vivo. We apply both sensors to visualize kinetics of bacterial labile manganese pools. Biophysical studies indicate the importance of coordinated solvent and hydrophobic interactions in the sensors’ selectivity. Our results establish lanmodulin as a versatile scaffold for design of selective protein-based biosensors and chelators for metals beyond the f-block.

