2023-04-27 デューク大学(Duke)
しかし、FDAはこの喉や鼻のスプレーを新しい製剤と見なし、従来から承認されている錠剤よりも濃度が数百万倍低いにも関わらず、一からのテストが必要だと考えていました。
Needhamは、ニクロサミドが既に承認されている製剤から十分な量を抽出できることを示すことで、テストと承認プロセスを迅速化することを望んでいます。
<関連情報>
- https://pratt.duke.edu/about/news/extracting-potent-covid-fighting-pharmaceuticals-protective-sprays
- https://aapsopen.springeropen.com/articles/10.1186/s41120-023-00072-x
市販の錠剤からニクロサミドを水性緩衝液に抽出し、COVID-19などの呼吸器感染症に対する経口・点鼻スプレーとして承認される可能性がある。 Extraction of niclosamide from commercial approved tablets into aqueous buffered solution creates potentially approvable oral and nasal sprays against COVID-19 and other respiratory infections
David Needham
AAPS Open Published:14 April 2023
DOI:https://doi.org/10.1186/s41120-023-00072-x
Abstract
Motivation
The low solubility, weak acid drug, niclosamide is a host cell modulator with broad-spectrum anti-viral cell-activity against many viruses, including stopping the SARS-CoV-2 virus from infecting cells in cell culture. As a result, a simple universal nasal spray preventative was proposed and investigated in earlier work regarding the dissolution of niclosamide into simple buffers. However, starting with pharmaceutical grade, niclosamide represents a new 505(b)(2) application. The motivation for this second paper in the series was therefore to explore if and to what extent niclosamide could be extracted from commercially available and regulatory-approved niclosamide oral tablets that could serve as a preventative nasal spray and an early treatment oral/throat spray, with possibly more expeditious testing and regulatory approval.
Experimental
Measurements of supernatant niclosamide concentrations were made by calibrated UV-Vis for the dissolution of niclosamide from commercially available Yomesan crushed into a powder for dissolution into Tris Buffer (TB) solutions. Parameters tested were as follows: time (0–2 days), concentration (300 µM to -1 mM), pH (7.41 to 9.35), and anhydrous/hydrated state. Optical microscopy was used to view the morphologies of the initial crushed powder, and the dissolving and equilibrating undissolved excess particles to detect morphologic changes that might occur.
Results
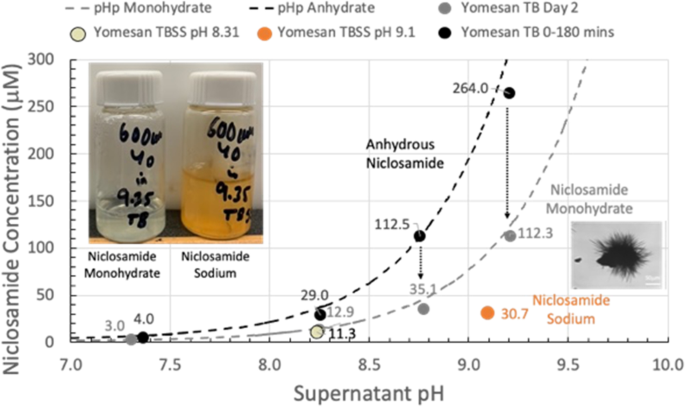
Concentration dependence: Niclosamide was readily extracted from powdered Yomesan at pH 9.34 TB at starting Yomesan niclosamide equivalents concentrations of 300 µM, 600 µM, and 1 mM. Peak dissolved niclosamide supernatant concentrations of 264 µM, 216 µM, and 172 µM were achieved in 1 h, 1 h, and 3 h respectively. These peaks though were followed by a reduction in supernatant concentration to an average of 112.3 µM ± 28.4 µM after overnight stir on day 2. pH dependence: For nominal pHs of 7.41, 8.35, 8.85, and 9.35, peak niclosamide concentrations were 4 µM, 22.4 µM, 96.2 µM, and 215.8 µM, respectively. Similarly, the day 2 values all reduced to 3 µM, 12.9 µM, 35.1 µM, and 112.3 µM. A heat-treatment to 200 °C dehydrated the niclosamide and showed a high 3 h concentration (262 µM) and the least day-2 reduction (to 229 µM). This indicated that the presence, or formation during exposure to buffer, of lower solubility polymorphs was responsible for the reductions in total solubilities. These morphologic changes were confirmed by optical microscopy that showed initially featureless particulate-aggregates of niclosamide could grow multiple needle-shaped crystals and form needle masses, especially in the presence of Tris-buffered sodium chloride, where new red needles were rapidly made. Scale up: A scaled-up 1 L solution of niclosamide was made achieving 165 µM supernatant niclosamide in 3 h by dissolution of just one fifth (100 mg niclosamide) of a Yomesan tablet.
Conclusion
These comprehensive results provide a guide as to how to utilize commercially available and approved tablets of niclosamide to generate aqueous niclosamide solutions from a simple dissolution protocol. As shown here, just one 4-tablet pack of Yomesan could readily make 165 L of a 20 µM niclosamide solution giving 16,500 10 mL bottles. One million bottles, from just 60 packs of Yomesan, would provide 100 million single spray doses for distribution to mitigate a host of respiratory infections as a universal preventative-nasal and early treatment oral/throat sprays throughout the world.
Graphical Abstract
pH dependence of niclosamide extraction from crushed Yomesan tablet material into Tris buffer (yellow-green in vial) and Tris-buffered saline solution (orange-red in vial). Initial anhydrous dissolution concentration is reduced by overnight stirring to likely monohydrate niclosamide; and is even lower if in TBSS forming new niclosamide sodium needle crystals grown from the original particles.