2023-07-10 ワシントン大学セントルイス校
◆研究では、インフリキシマブやアバタセプトなどの薬剤を標準治療に追加した場合の効果を調査しました。これらの薬剤は死亡率を低下させる効果があり、治療ガイドラインの改定に役立つ可能性があります。しかし、依然としてCOVID-19の重症患者に対する治療法の選択肢は広がっています。
<関連情報>
- https://source.wustl.edu/2023/07/anti-inflammatory-drugs-did-not-speed-covid-19-recovery-but-prevented-deaths/
- https://jamanetwork.com/journals/jama/fullarticle/2807333
COVID-19肺炎で入院した成人の治療に対するアバタセプト、セニクリビロク、またはインフリキシマブ 無作為臨床試験 Abatacept, Cenicriviroc, or Infliximab for Treatment of Adults Hospitalized With COVID-19 Pneumonia A Randomized Clinical Trial
Jane A. O’Halloran, Emily R. Ko, Kevin J. Anstrom, Eyal Kedar, Matthew W. McCarthy, Reynold A. Panettieri Jr, Martin Maillo, Patricia Segura Nunez, Anne M . Lachiewicz, Cynthia Gonzalez, Brian Smith, Sabina Mendivil-Tuchia de Tai, Akram Khan, Alfredo J. Mena Lora, Matthias Salathe, Gerardo Capo, Daniel Rodríguez Gonzalez, Thomas F. Patterson, Christopher Palma, Horacio Ariza, Maria Patelli Lima, John Blamoun, Esteban C. Nannini, Eduardo Sprinz, Analia Mykietiuk, Radica Alicic, Adriana M. Rauseo, Cameron R. Wolfe, Britta Witting; Jennifer P. Wang, Luis Parra-Rodriguez, Tatyana Der, Kate Willsey, Jun Wen, Adam Silverstein, Sean M. O’Brien, Hussein R. Al-Khalidi, Michael A. Maldonado, Richard Melsheimer; William G. Ferguson, Steven E. McNulty, Pearl Zakroysky, Susan Halabi, Daniel K. Benjamin Jr, Sandra Butler, Jane C. Atkinson, Stacey J. Adam, Soju Chang, Lisa LaVange, Michael Proschan, Samuel A. Bozzette, William G. Powderly, for the ACTIV-1 IM Study Group Members
JAMA Published: July 10, 2023
DOI:10.1001/jama.2023.11043
Key Points
Question Do abatacept, cenicriviroc, or infliximab improve time to recovery for patients hospitalized with COVID-19 pneumonia compared with standard care?
Findings A randomized, double-masked, placebo-controlled master protocol clinical trial found that treatment with abatacept, cenicriviroc, or infliximab showed no statistically significant difference for the primary end point of time to recovery in patients with COVID-19 pneumonia vs standard care.
Meaning Abatacept, cenicriviroc, or infliximab did not decrease time to recovery for hospitalized patients with COVID-19 pneumonia.
Abstract
Importance Immune dysregulation contributes to poorer outcomes in COVID-19.
Objective To investigate whether abatacept, cenicriviroc, or infliximab provides benefit when added to standard care for COVID-19 pneumonia.
Design, Setting, and Participants Randomized, double-masked, placebo-controlled clinical trial using a master protocol to investigate immunomodulators added to standard care for treatment of participants hospitalized with COVID-19 pneumonia. The results of 3 substudies are reported from 95 hospitals at 85 clinical research sites in the US and Latin America. Hospitalized patients 18 years or older with confirmed SARS-CoV-2 infection within 14 days and evidence of pulmonary involvement underwent randomization between October 2020 and December 2021.
Interventions Single infusion of abatacept (10 mg/kg; maximum dose, 1000 mg) or infliximab (5 mg/kg) or a 28-day oral course of cenicriviroc (300-mg loading dose followed by 150 mg twice per day).
Main Outcomes and Measures The primary outcome was time to recovery by day 28 evaluated using an 8-point ordinal scale (higher scores indicate better health). Recovery was defined as the first day the participant scored at least 6 on the ordinal scale.
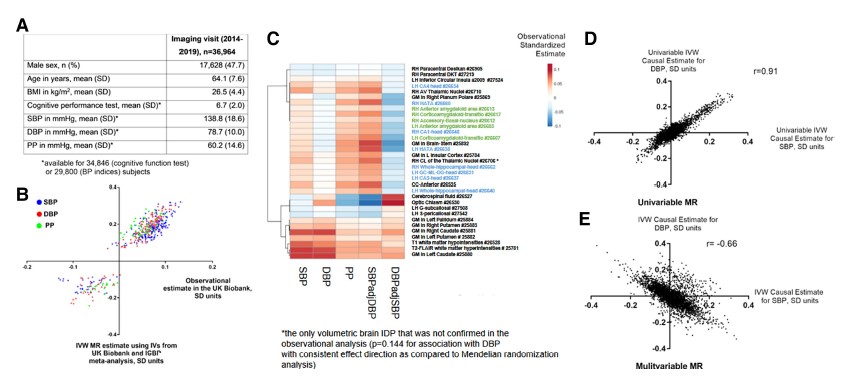
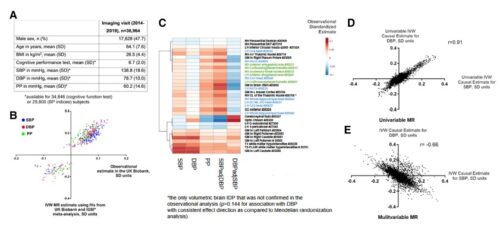
Results Of the 1971 participants randomized across the 3 substudies, the mean (SD) age was 54.8 (14.6) years and 1218 (61.8%) were men. The primary end point of time to recovery from COVID-19 pneumonia was not significantly different for abatacept (recovery rate ratio [RRR], 1.12 [95% CI, 0.98-1.28]; P = .09), cenicriviroc (RRR, 1.01 [95% CI, 0.86-1.18]; P = .94), or infliximab (RRR, 1.12 [95% CI, 0.99-1.28]; P = .08) compared with placebo. All-cause 28-day mortality was 11.0% for abatacept vs 15.1% for placebo (odds ratio [OR], 0.62 [95% CI, 0.41-0.94]), 13.8% for cenicriviroc vs 11.9% for placebo (OR, 1.18 [95% CI 0.72-1.94]), and 10.1% for infliximab vs 14.5% for placebo (OR, 0.59 [95% CI, 0.39-0.90]). Safety outcomes were comparable between active treatment and placebo, including secondary infections, in all 3 substudies.
Conclusions and Relevance Time to recovery from COVID-19 pneumonia among hospitalized participants was not significantly different for abatacept, cenicriviroc, or infliximab vs placebo.
Trial Registration ClinicalTrials.gov Identifier: NCT04593940