2024-02-07 マサチューセッツ工科大学(MIT)
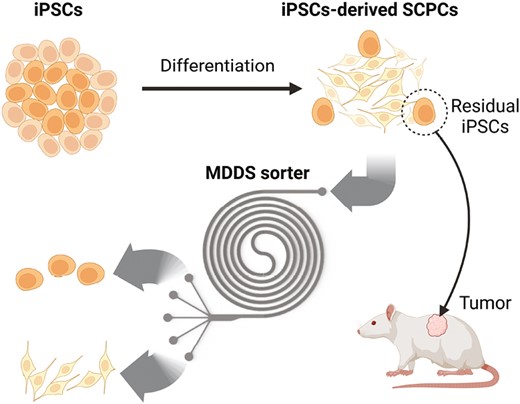
◆このデバイスは、細胞治療において脊髄損傷を治療するために、皮膚や血液細胞からiPS細胞を作成し、それらを脊髄細胞へと分化させる。しかし、完全に分化しないiPS細胞は腫瘍を形成する可能性があり、このチームはその半数を取り除くマイクロ流体セルソーターを開発した。このデバイスは、特殊な化学物質を必要とせず、1分間に300万以上の細胞をソートできる高スループットデバイスであり、低コストで量産可能である。
<関連情報>
- https://news.mit.edu/2024/scientists-develop-low-cost-device-safer-cell-therapy-0207
- https://academic.oup.com/stcltm/advance-article/doi/10.1093/stcltm/szae002/7597591?login=false
iPSC由来の脊髄前駆細胞から残存未分化細胞をラベルフリーでハイスループットに除去 Label-Free and High-Throughput Removal of Residual Undifferentiated Cells From iPSC-Derived Spinal Cord Progenitor Cells
Tan Dai Nguyen, Wai Hon Chooi, Hyungkook Jeon, Jiahui Chen, Jerome Tan, Daniel N Roxby, Cheryl Yi-Pin Lee, Shi-Yan Ng, Sing Yian Chew, Jongyoon Han
Stem Cells Translational Medicine Published:07 February 2024
DOI:https://doi.org/10.1093/stcltm/szae002
Abstract
The transplantation of spinal cord progenitor cells (SCPCs) derived from human-induced pluripotent stem cells (iPSCs) has beneficial effects in treating spinal cord injury (SCI). However, the presence of residual undifferentiated iPSCs among their differentiated progeny poses a high risk as these cells can develop teratomas or other types of tumors post-transplantation. Despite the need to remove these residual undifferentiated iPSCs, no specific surface markers can identify them for subsequent removal. By profiling the size of SCPCs after a 10-day differentiation process, we found that the large-sized group contains significantly more cells expressing pluripotent markers. In this study, we used a sized-based, label-free separation using an inertial microfluidic-based device to remove tumor-risk cells. The device can reduce the number of undifferentiated cells from an SCPC population with high throughput (ie, >3 million cells/minute) without affecting cell viability and functions. The sorted cells were verified with immunofluorescence staining, flow cytometry analysis, and colony culture assay. We demonstrated the capabilities of our technology to reduce the percentage of OCT4-positive cells. Our technology has great potential for the “downstream processing” of cell manufacturing workflow, ensuring better quality and safety of transplanted cells.
Graphical Abstract