2024-02-08 カリフォルニア大学サンディエゴ校(UCSD)
◆UCサンディエゴの科学者らは、mtDNAがミトコンドリア外に排出される新しいメカニズムを発見し、これが炎症を引き起こす免疫経路を活性化することを明らかにした。この発見は、炎症性疾患の治療法の開発に新たな可能性を提供する。
<関連情報>
- https://today.ucsd.edu/story/faulty-dna-disposal-system-causes-inflammation
- https://www.nature.com/articles/s41556-023-01343-1
ミトコンドリアDNA複製ストレスが、炎症性エンドソームのヌクレオイド廃棄経路を引き起こす Mitochondrial DNA replication stress triggers a pro-inflammatory endosomal pathway of nucleoid disposal
Laura E. Newman,Sammy Weiser Novak,Gladys R. Rojas,Nimesha Tadepalle,Cara R. Schiavon,Danielle A. Grotjahn,Christina G. Towers,Marie-Ève Tremblay,Matthew P. Donnelly,Sagnika Ghosh,Michaela Medina,Sienna Rocha,Ricardo Rodriguez-Enriquez,Joshua A. Chevez,Ian Lemersal,Uri Manor & Gerald S. Shadel
Nature Cell Biology Published:08 February 2024
DOI:https://doi.org/10.1038/s41556-023-01343-1
Abstract
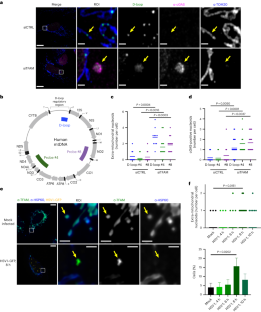
Mitochondrial DNA (mtDNA) encodes essential subunits of the oxidative phosphorylation system, but is also a major damage-associated molecular pattern (DAMP) that engages innate immune sensors when released into the cytoplasm, outside of cells or into circulation. As a DAMP, mtDNA not only contributes to anti-viral resistance, but also causes pathogenic inflammation in many disease contexts. Cells experiencing mtDNA stress caused by depletion of the mtDNA-packaging protein, transcription factor A, mitochondrial (TFAM) or during herpes simplex virus-1 infection exhibit elongated mitochondria, enlargement of nucleoids (mtDNA–protein complexes) and activation of cGAS–STING innate immune signalling via mtDNA released into the cytoplasm. However, the relationship among aberrant mitochondria and nucleoid dynamics, mtDNA release and cGAS–STING activation remains unclear. Here we show that, under a variety of mtDNA replication stress conditions and during herpes simplex virus-1 infection, enlarged nucleoids that remain bound to TFAM exit mitochondria. Enlarged nucleoids arise from mtDNA experiencing replication stress, which causes nucleoid clustering via a block in mitochondrial fission at a stage when endoplasmic reticulum actin polymerization would normally commence, defining a fission checkpoint that ensures mtDNA has completed replication and is competent for segregation into daughter mitochondria. Chronic engagement of this checkpoint results in enlarged nucleoids trafficking into early and then late endosomes for disposal. Endosomal rupture during transit through this endosomal pathway ultimately causes mtDNA-mediated cGAS–STING activation. Thus, we propose that replication-incompetent nucleoids are selectively eliminated by an adaptive mitochondria–endosomal quality control pathway that is prone to innate immune system activation, which might represent a therapeutic target to prevent mtDNA-mediated inflammation during viral infection and other pathogenic states.