2024-04-24 エディンバラ大学
<関連情報>
- https://www.ed.ac.uk/news/2024/new-method-could-cut-drug-production-waste
- https://www.nature.com/articles/s41557-024-01504-1
立体規則性エナンチオ変換反応 Stereoretentive enantioconvergent reactions
Steven H. Bennett,Jacob S. Bestwick,Vera P. Demertzidou,David J. Jones,Helen E. Jones,François Richard,Joshua A. Homer,Rosie Street-Jeakings,Andrew F. Tiberia & Andrew L. Lawrence
Nature Chemistry Published:17 April 2024
DOI:https://doi.org/10.1038/s41557-024-01504-1
Abstract
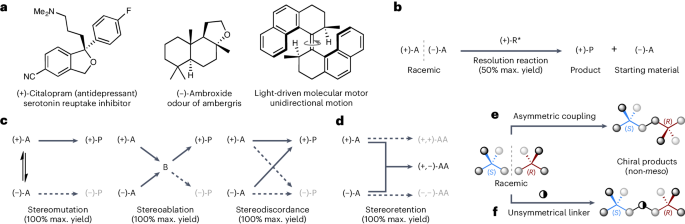
Enantioconvergent reactions are pre-eminent in contemporary asymmetric synthesis as they convert both enantiomers of a racemic starting material into a single enantioenriched product, thus avoiding the maximum 50% yield associated with resolutions. All currently known enantioconvergent processes necessitate the loss or partial loss of the racemic substrate’s stereochemical information, thus limiting the potential substrate scope to molecules that contain labile stereogenic units. Here we present an alternative approach to enantioconvergent reactions that can proceed with full retention of the racemic substrate’s configuration. This uniquely stereo-economic approach is possible if the two enantiomers of a racemic starting material are joined together to form one enantiomer of a non-meso product. Experimental validation of this concept is presented using two distinct strategies: (1) a direct asymmetric coupling approach, and (2) a multicomponent approach, which exhibits statistical amplification of enantiopurity. Thus, the established dogma that enantioconvergent reactions require substrates that contain labile stereogenic units is shown to be incorrect.


