2024-05-10 バッファロー大学(UB)
<関連情報>
- https://www.buffalo.edu/news/releases/2024/05/Ranibizumab-safe-for-retinopathy-of-prematurity.html
- https://www.sciencedirect.com/science/article/pii/S2589537024001469
未熟児網膜症の超低出生体重児に対するラニビズマブとレーザー治療の比較(RAINBOW):無作為化試験の5年間の成績 Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): five-year outcomes of a randomised trial
Neil Marlow, James D. Reynolds, Domenico Lepore, Alistair R. Fielder, Andreas Stahl, Han Hao, Annemarie Weisberger, Amit Lodha, Brian W. Fleck
eClinicalMedicine Available online:11 April 2024
DOI:https://doi.org/10.1016/j.eclinm.2024.102567

Summary
Background
Concerns remain over the long-term safety of vascular endothelial growth factor (VEGF) inhibitors to treat retinopathy of prematurity (ROP). RAINBOW is an open label randomised trial comparing intravitreal ranibizumab (in 0.2 mg and 0.1 mg doses) with laser therapy in very low birthweight infants (<1500 g) with ROP.
Methods
Of 201 infants completing RAINBOW, 180 were enrolled in the RAINBOW Extension Study. At 5 years, children underwent ophthalmic, development and health assessments. The primary outcome was visual acuity in the better-seeing eye. The study is registered with ClinicalTrial.gov, NCT02640664.
Findings
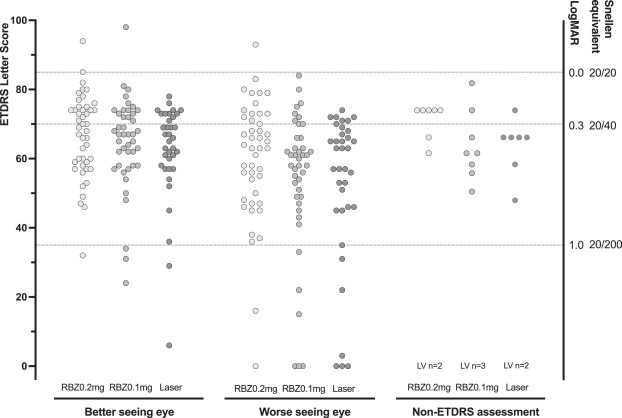
Between 16-6-2016 and 21-4-2022, 156 children (87%) were evaluated at 5 years. Of 32 children with no acuity test result, 25 had a preferential looking test, for 4 children investigators reported low vision for each eye, and in 3 further children no vision measurement was obtained. 124 children completed the acuity assessment, the least square mean (95% CI) letter score in the better seeing eye was similar in the three trial arms—66.8 (62.9–70.7) following ranibizumab 0.2 mg, 64.6 (60.6–68.5) following ranibizumab 0.1 mg and 62.1 (57.8–66.4) following laser therapy; differences in means: ranibizumab 0.2 mg v laser: 4.7 (95% CI: -1.1, 10.5); 0.1 mg v laser: 2.5 (-3.4, 8.3); 0.2 mg v 0.1 mg: 2.2 (-3.3, 7.8). High myopia (worse than -5 dioptres) in at least one eye occurred in 4/52 (8%) children following ranibizumab 0.2 mg, 8/55 (15%) following ranibizumab 0.1 mg and 11/45 (24%) following laser therapy (0.2 mg versus laser: odds ratio: 3.99 (1.16–13.72)). Ocular and systemic secondary outcomes and adverse events were distributed similarly in each trial arm.
Interpretation
5-year outcomes confirm the findings of the original RAINBOW trial and a planned interim analysis at 2 years, including a reduced frequency of high myopia following ranibizumab treatment. No effects of treatment on non-ocular outcomes were detected.
Funding
Novartis Pharma AG.