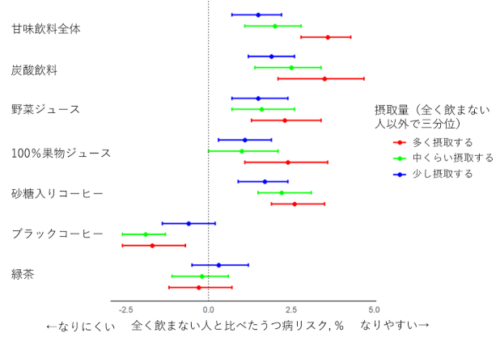
2024-06-05 ミュンヘン大学(LMU)
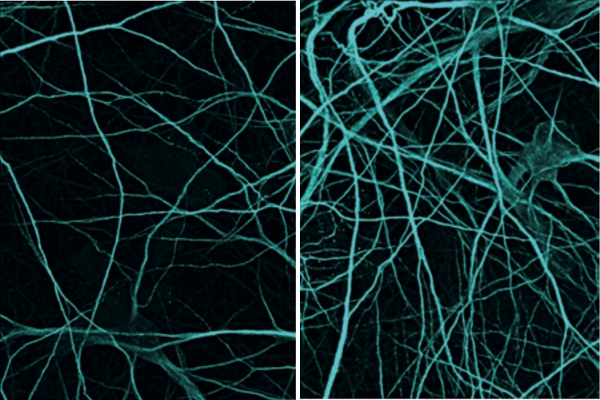
The researchers tested their novel method on cultured human nerve cells. Compared to before (left), significantly more nerve cells were present after the treatment (right). © Marvin Reich /Kultivierte men LMU & ISD
◆ミュンヘン大学(LMU)とドイツ神経変性疾患センター(DZNE)の研究者たちは、プログラニュリンを補充する新しい治療法を開発しました。この方法では、ウイルスを用いて肝細胞にプログラニュリンを大量生産させ、血液を通じて脳に届けることで、副作用を回避します。マウスモデルと幹細胞モデルの両方で症状の大幅な改善が確認されました。この研究は前臨床試験で有望な結果を示し、今後の臨床応用が期待されます。
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/frontotemporal-dementia-therapeutic-approach-for-gene-therapy.html
- https://www.science.org/doi/10.1126/scitranslmed.adj7308
脳内浸透性プログラニュリンの末梢発現が前頭側頭葉変性症モデルマウスの病態を救う Peripheral expression of brain-penetrant progranulin rescues pathologies in mouse models of frontotemporal lobar degeneration
MARVIN REICH, MATTHEW J. SIMON, BEATE POLKE, IÑAKI PARIS, […], AND CHRISTIAN HAASS
Science Translational Medicine Published:5 Jun 2024
DOI:https://doi.org/10.1126/scitranslmed.adj7308
Editor’s summary
The restoration of brain progranulin holds promise for the treatment of frontotemporal lobar degeneration with GRN loss-of-function mutations (FTLD-GRN). However, delivering therapeutics to the brain remains a challenge. Here, Reich et al. developed an adeno-associated virus (AAV) vector for the sustained expression of a brain-penetrant variant of human progranulin in the liver. Using two mouse models of FTLD-GRN, the authors show that a single intravenous injection of this AAV achieved sustained progranulin concentrations in the brain for up to 31 weeks and could rescue different FTLD-GRN associated pathologies, highlighting the translational potential of this approach. —Daniela Neuhofer
Abstract
Progranulin (PGRN) haploinsufficiency is a major risk factor for frontotemporal lobar degeneration with TAR DNA-binding protein 43 (TDP-43) pathology (FTLD-GRN). Multiple therapeutic strategies are in clinical development to restore PGRN in the CNS, including gene therapy. However, a limitation of current gene therapy approaches aimed to alleviate FTLD-associated pathologies may be their inefficient brain exposure and biodistribution. We therefore developed an adeno-associated virus (AAV) targeting the liver (L) to achieve sustained peripheral expression of a transferrin receptor (TfR) binding, brain-penetrant (b) PGRN variant [AAV(L):bPGRN] in two mouse models of FTLD-GRN, namely, Grn knockout and GrnxTmem106b double knockout mice. This therapeutic strategy avoids potential safety and biodistribution issues of CNS-administered AAVs and maintains sustained concentrations of PGRN in the brain after a single dose. AAV(L):bPGRN treatment reduced several FTLD-GRN–associated pathologies including severe motor function deficits, aberrant TDP-43 phosphorylation, dysfunctional protein degradation, lipid metabolism, gliosis, and neurodegeneration in the brain. The potential translatability of our findings was tested in an in vitro model using cocultured human induced pluripotent stem cell (hiPSC)–derived microglia lacking PGRN and TMEM106B and wild-type hiPSC-derived neurons. As in mice, aberrant TDP-43, lysosomal dysfunction, and neuronal loss were ameliorated after treatment with exogenous TfR-binding protein transport vehicle fused to PGRN (PTV:PGRN). Together, our studies suggest that peripherally administered brain-penetrant PGRN replacement strategies ameliorate FTLD-GRN relevant phenotypes including TDP-43 pathology, neurodegeneration, and behavioral deficits. Our data provide preclinical proof of concept for the use of this AAV platform for treatment of FTLD-GRN and potentially other CNS disorders.