20024-07-29 カリフォルニア大学サンディエゴ校(UCSD)
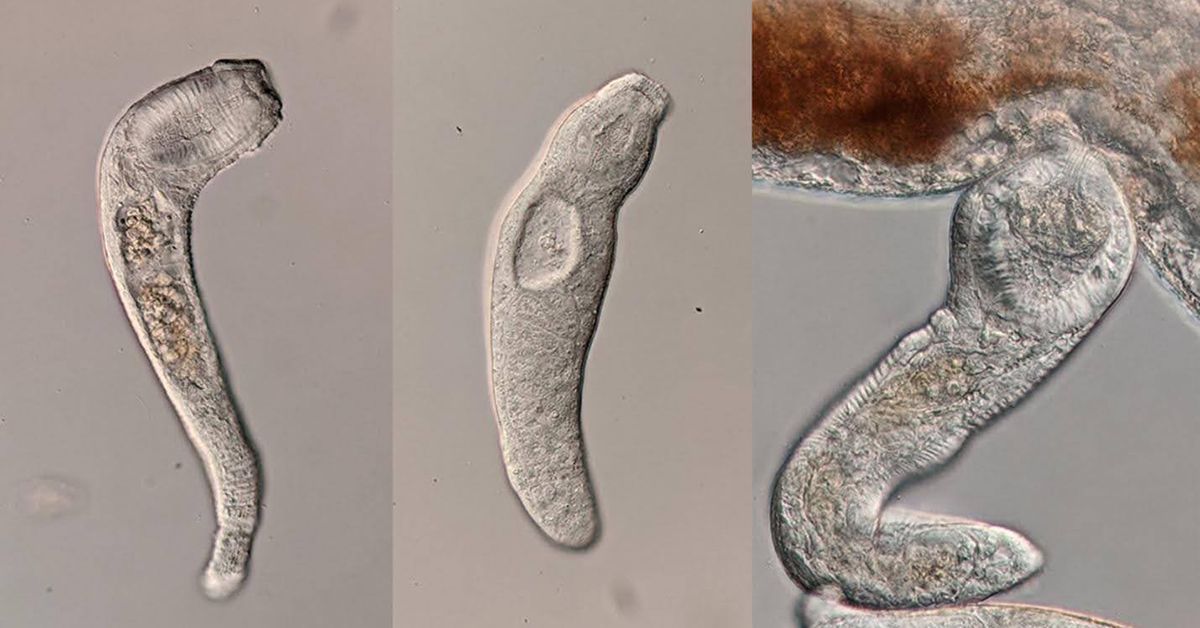 The parasitic flatworm Haplorchis pumilio produces non-reproductive soldiers (left) which have much larger mouths than their reproductively capable colony-mates (center). The soldiers use their mouths to defend the colony by producing powerful blasts of suction to kill their enemies (right). Credit: Dan Metz
The parasitic flatworm Haplorchis pumilio produces non-reproductive soldiers (left) which have much larger mouths than their reproductively capable colony-mates (center). The soldiers use their mouths to defend the colony by producing powerful blasts of suction to kill their enemies (right). Credit: Dan Metz
<関連情報>
- https://today.ucsd.edu/story/human-infecting-parasite-produces-sterile-soldiers-like-ants-and-termites
- https://www.pnas.org/doi/10.1073/pnas.2400953121
ヒトに感染する侵入性扁形虫の物理的な兵士カーストは、形態学的に極端であり、義務的に無菌である The physical soldier caste of an invasive, human-infecting flatworm is morphologically extreme and obligately sterile
Daniel C. G. Metz and Ryan F. Hechinger
Proceedings of the National Academy of Sciences Published:July 23, 2024
DOI:https://doi.org/10.1073/pnas.2400953121
Significance
We provide substantial evidence that a globally invasive, human-infectious trematode (parasitic flatworm) in its first intermediate host possesses a physically specialized and obligately sterile soldier caste. Unlike similar-sized immature reproductives, soldier worms both lack reproductive organs and attack and kill competitor trematodes. The soldiers also possess the most extreme morphological specialization for defense yet observed in trematodes, having a giant muscular pharynx analogous to the enlarged mandibles of soldier ants. Partly due to the effectiveness of its specialized soldiers in killing competitors, Haplorchis pumilio substantially structures the ecological community of trematodes infecting its invasive host snail. This work indicates that obligate sterility, akin to the complete division of labor in the social insects, has independently evolved in the flatworms.
Abstract
We show that the globally invasive, human-infectious flatworm, Haplorchis pumilio, possesses the most physically specialized soldier caste yet documented in trematodes. Soldiers occur in colonies infecting the first intermediate host, the freshwater snail Melanoides tuberculata, and are readily distinguishable from immature and mature reproductive worms. Soldiers possess a pharynx five times absolutely larger than those of immature and mature reproductives, lack a germinal mass, and have a different developmental trajectory than reproductives, indicating that H. pumilio soldiers constitute a reproductively sterile physical caste. Neither immature nor mature reproductives showed aggression in in vitro trials, but soldiers readily attacked heterospecific trematodes that coinfect their host. Ecologically, we calculate that H. pumilio caused ~94% of the competitive deaths in the guild of trematodes infecting its host snail in its invasive range in southern California. Despite being a dominant competitor, H. pumilio soldiers did not attack conspecifics from other colonies. All prior reports documenting division of labor and a trematode soldier caste have involved soldiers that may be able to metamorphose to the reproductive stage and have been from nonhuman-infectious marine species; this study provides clear evidence for an obligately sterile trematode soldier, while extending the phenomenon of a trematode soldier caste to freshwater and to an invasive species of global public health concern.