2024-08-06 ワシントン大学セントルイス校
<関連情報>
- https://source.wustl.edu/2024/08/dna-fragments-help-detect-kidney-organ-rejection/
- https://www.nature.com/articles/s41591-024-03087-3
無細胞DNAによる腎移植片拒絶反応の検出 Cell-free DNA for the detection of kidney allograft rejection
Olivier Aubert,Cindy Ursule-Dufait,Romain Brousse,Juliette Gueguen,Maud Racapé,Marc Raynaud,Elisabet Van Loon,Angelica Pagliazzi,Edmund Huang,Stanley C. Jordan,Kenneth D. Chavin,Gaurav Gupta,Dhiren Kumar,Tarek Alhamad,Sanjiv Anand,Jorge Sanchez-Garcia,Basmah A. Abdalla,Julien Hogan,Rouba Garro,Darshana M. Dadhania,Pranjal Jain,Didier A. Mandelbrot,Maarten Naesens,Raja Dandamudi,… Alexandre Loupy
Nature Medicine Published:02 June 2024
DOI:https://doi.org/10.1038/s41591-024-03087-3
Abstract
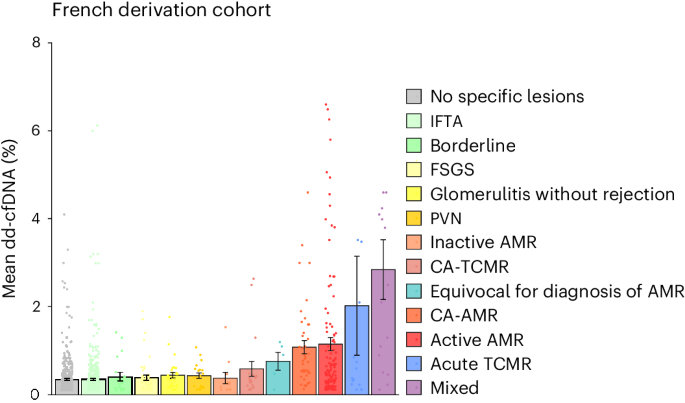
Donor-derived cell-free DNA (dd-cfDNA) is an emerging noninvasive biomarker that has the potential to detect allograft injury. The capacity of dd-cfDNA to detect kidney allograft rejection and its added clinical value beyond standard of care patient monitoring is unclear. We enrolled 2,882 kidney allograft recipients from 14 transplantation centers in Europe and the United States in an observational population-based study. The primary analysis included 1,134 patients. Donor-derived cell-free DNA levels strongly correlated with allograft rejection, including antibody-mediated rejection (P < 0.0001), T cell-mediated rejection (P < 0.0001) and mixed rejection (P < 0.0001). In multivariable analysis, circulating dd-cfDNA was significantly associated with allograft rejection (odds ratio 2.275; 95% confidence interval (CI) 1.902–2.739; P < 0.0001) independently of standard of care patient monitoring parameters. The inclusion of dd-cfDNA to a standard of care prediction model showed improved discrimination (area under the curve 0.777 (95% CI 0.741–0.811) to 0.821 (95% CI 0.784–0.852); P = 0.0011) and calibration. These results were confirmed in the external validation cohorts (n = 1,748) including a cohort of African American patients (n = 439). Finally, dd-cfDNA showed high predictive value to detect subclinical rejection in stable patients. Our study provides insights on the potential value of assessing dd-cfDNA, in addition to standard of care monitoring, to improve the detection of allograft rejection. ClinicalTrials.gov registration: NCT05995379.