2025-01-14 カリフォルニア大学サンディエゴ校(UCSD)
<関連情報>
- https://today.ucsd.edu/story/genetic-tweak-optimizes-drug-making-cells-by-blocking-buildup-of-toxic-byproduct
- https://www.nature.com/articles/s42255-024-01193-7
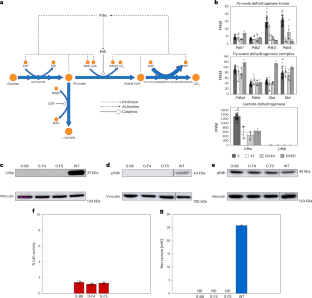
マルチプレックスゲノム編集により、哺乳類細胞の増殖速度に影響を与えることなく乳酸産生が除去される Multiplex genome editing eliminates lactate production without impacting growth rate in mammalian cells
Hooman Hefzi,Iván Martínez-Monge,Igor Marin de Mas,Nicholas Luke Cowie,Alejandro Gomez Toledo,Soo Min Noh,Karen Julie la Cour Karottki,Marianne Decker,Johnny Arnsdorf,Jose Manuel Camacho-Zaragoza,Stefan Kol,Sanne Schoffelen,Nuša Pristovšek,Anders Holmgaard Hansen,Antonio A. Miguez,Sara Petersen Bjørn,Karen Kathrine Brøndum,Elham Maria Javidi,Kristian Lund Jensen,Laura Stangl,Emanuel Kreidl,Thomas Beuchert Kallehauge,Daniel Ley,Patrice Ménard,… Nathan E. Lewis
Nature Metabolism Published:14 January 2025
DOI:https://doi.org/10.1038/s42255-024-01193-7
Abstract
The Warburg effect, which describes the fermentation of glucose to lactate even in the presence of oxygen, is ubiquitous in proliferative mammalian cells, including cancer cells, but poses challenges for biopharmaceutical production as lactate accumulation inhibits cell growth and protein production. Previous efforts to eliminate lactate production in cells for bioprocessing have failed as lactate dehydrogenase is essential for cell growth. Here, we effectively eliminate lactate production in Chinese hamster ovary and in the human embryonic kidney cell line HEK293 by simultaneous knockout of lactate dehydrogenases and pyruvate dehydrogenase kinases, thereby removing a negative feedback loop that typically inhibits pyruvate conversion to acetyl-CoA. These cells, which we refer to as Warburg-null cells, maintain wild-type growth rates while producing negligible lactate, show a compensatory increase in oxygen consumption, near total reliance on oxidative metabolism, and higher cell densities in fed-batch cell culture. Warburg-null cells remain amenable for production of diverse biotherapeutic proteins, reaching industrially relevant titres and maintaining product glycosylation. The ability to eliminate lactate production may be useful for biotherapeutic production and provides a tool for investigating a common metabolic phenomenon.