2025-02-26 中国科学院 (CAS)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202502/t20250226_902628.shtml
- https://www.nature.com/articles/s41467-025-57287-7
GORK K+チャネルの構造とゲーティングが気孔工学に不可欠であることを発見 GORK K+ channel structure and gating vital to informing stomatal engineering
Xue Zhang,William Carroll,Thu Binh-Anh Nguyen,Thanh-Hao Nguyen,Zhao Yang,Miaolian Ma,Xiaowei Huang,Adrian Hills,Hui Guo,Rucha Karnik,Michael R. Blatt & Peng Zhang
Nature Communications Published:25 February 2025
DOI:https://doi.org/10.1038/s41467-025-57287-7
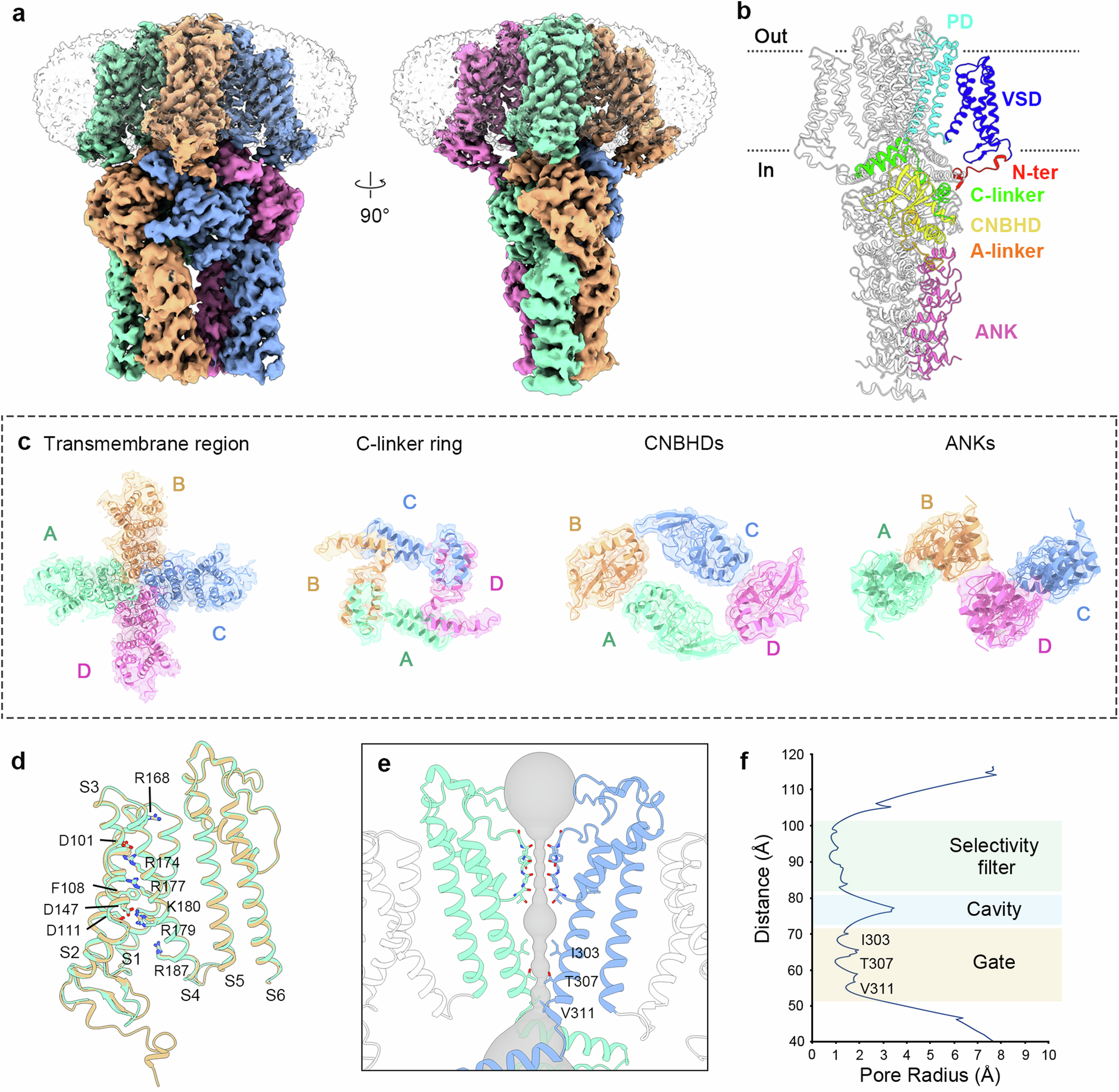
Abstract
The Arabidopsis GORK channel is a major pathway for guard cell K+ efflux that facilitates stomatal closure. GORK is an outwardly-rectifying member of the cyclic-nucleotide binding-homology domain (CNBHD) family of K+ channels with close homologues in all other angiosperms known to date. Its bioengineering has demonstrated the potential for enhanced carbon assimilation and water use efficiency. Here we identify critical domains through structural and functional analysis, highlighting conformations that reflect long-lived closed and pre-open states of GORK. These conformations are marked by interactions at the cytosolic face of the membrane between so-called voltage-sensor, C-linker and CNBHD domains, the latter relocating across 10 Å below the voltage sensor. The interactions center around two coupling sites that functional analysis establish are critical for channel gating. The channel is also subject to putative, ligand-like interactions within the CNBHD, which leads to its gating independence of cyclic nucleotides such as cAMP or cGMP. These findings implicate a multi-step mechanism of semi-independent conformational transitions that underlie channel activity and offer promising new sites for optimizing GORK to engineer stomata.